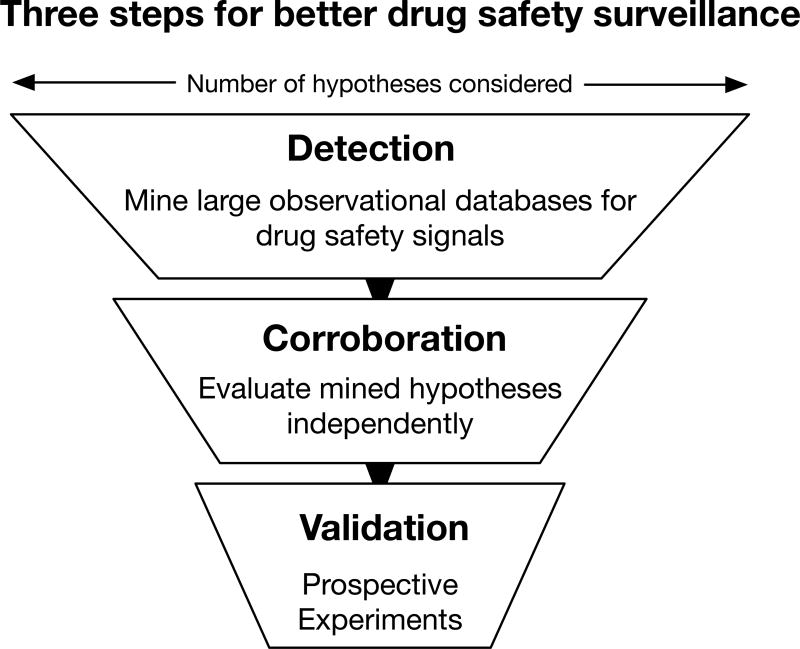
Figure 1. The three steps to more rigorous and efficient drugs safety surveillance.
Step 1. Detection. Modern signal detection and statistical data mining method are used to identify new drug safety and drug-drug interaction signals. Methods include traditional approaches, like disproportionality analysis with statistical corrections, and newer methods, like supervised machine learning and pattern detection. This step produces a lot of statistically significant associations but also a lot of false discoveries. Step 2. Corroboration. Mined hypotheses are evaluated against an independent dataset and evaluated for plausibility. These additional data could score drug effect hypotheses by their molecular connection to the known effect or provide additional clinical evidence from alternative resources. Any hypotheses that does not corroborate is removed from consideration, greatly reduces the number of false discoveries. At the end of this stage only a few to dozens of the strongest hypotheses remain. Step 3. Validation. Corroborated hypotheses are validated using a model system. Model systems may be molecular assays (e.g. chemical-protein binding affinity), cellular systems, or animal models, depending on which model is best suited for the predicted adverse reaction outcome. Drug effects that validate in all three steps have demonstrated clinical importance in humans (steps 1 and 2) and have evidence of causality (step 3). This process is both more efficient than a clinical trial and more rigorous than a retrospective analysis.