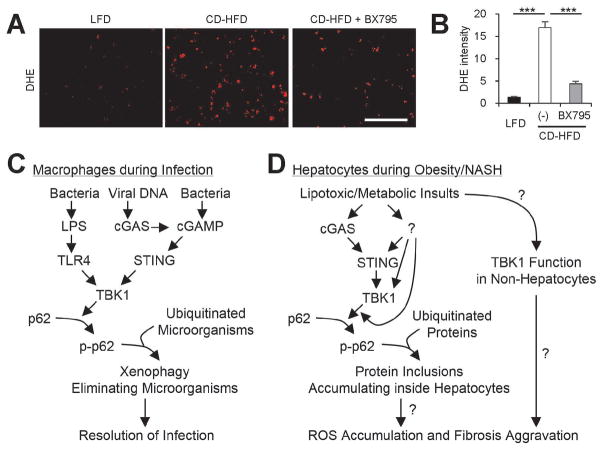
Fig. 8.
Inhibition of TBK1 suppresses hepatic oxidative stress during NASH. (A) Fresh frozen liver sections from mice described in Fig. 6 were analyzed by dihydroethidium (DHE) staining, which visualizes reactive oxygen species (ROS). Scale bars, 200 μm. (B) DHE fluorescence intensity was quantified. Data are shown as mean ± s.e.m. ***P < 0.001 (Student’s t-test). (C) During infection, TBK1-dependent p62 phosphorylation induces aggregation of ubiquitinated microorganisms, making them better substrates for autophagy, which leads to quicker microorganism elimination. (D) During obesity and NAFLD, lipotoxic activation of TBK1 signaling provokes the formation of p62-ubiquitin aggregates. Due to autophagy defects, hepatocytes cannot efficiently eliminate these aggregates, leading to the formation of large protein inclusions during NASH. These protein inclusions can provoke sublethal ROS accumulation, which can culminate in the development of fibrosis during NASH. In addition, lipotoxicity may also increase TBK1 signaling in non-hepatocytes, such as hepatic stellate cells and immune cells, which may contribute to the fibrotic pathologies. Question marks denote the mechanistic connections that still need to be established by future investigations.