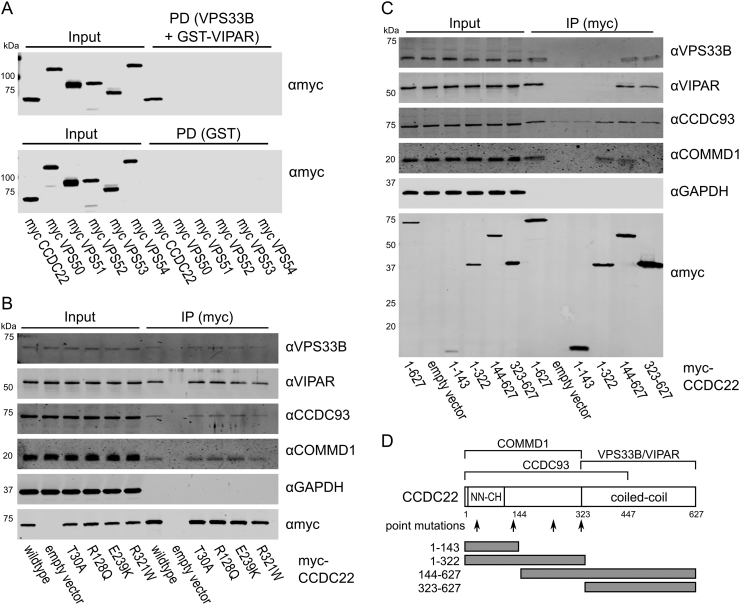
Fig. 3.
The C-terminal coiled-coil region of CCDC22 binds directly to VPS33B/GST-VIPAR. (A) Myc-tagged CCDC22 or GARP and EARP subunits were produced by in vitro transcription/translation and then subjected to GST pull-down (PD) using VPS33B/GST-VIPAR or GST alone. Samples were analyzed by immunoblotting with anti-myc. CCDC22 is efficiently pulled down by VPS33B/GST-VIPAR, but no GARP or EARP subunits are pulled down. (B) Myc-tagged CCDC22 with the wild-type sequence or clinically relevant point mutations [44], [45], or the equivalent amount of empty vector, were transfected into HEK293T cells. After immunoprecipitation (IP), samples were immunoblotted for endogenous proteins using the antibodies indicated. (C) Full length (1–627) and truncated myc-CCDC22, or the equivalent amount of empty vector, were transfected into HEK293T cells. After IP, samples were immunoblotted for endogenous proteins using the antibodies indicated. (D) Schematic of CCDC22, showing predicted N-terminal calponin homology (CH)-like (NN-CH) and C-terminal coiled-coil domains [38], [45], [63], [64], regions required for binding to COMMD1 and CCDC93 [38], [45], the region found to bind VPS33B/VIPAR in this study, and constructs used in this paper.