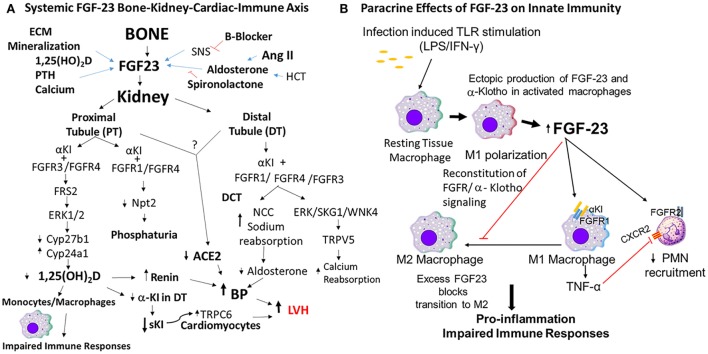
Figure 1.
Mechanisms of fibroblast growth factor-23 (FGF-23) effects to impair innate immune responses. (A) FGF-23 bone–kidney–cardiac–immune axis. FGF-23 is produced by osteoblasts/osteocytes in bone in response to local and systemic factors and targets the kidney to create multiple endocrine networks (4, 13) in bone, including (1) an FGF-23 vitamin D counter-regulatory loop (4, 14); (2) a calcium-FGF-23 endocrine loop, where calcium stimulates FGF-23 in bone and FGF-23 stimulates calcium reabsorption in the DT (15, 16); (3) a bone–kidney axis, where FGF-23 is regulated by factors involved in extracellular matrix mineralization to coordinate renal phosphate handling (1). In addition, there is a bone–renal–cardiac axis that augments hemodynamic responses through a feed forward bone–cardio–renal loop, where angiotensin II (Ang II) stimulates FGF-23 production in bone (17) and FGF-23 suppresses Ace2 in the kidney (18), a volume regulatory loop where diuretics and aldosterone stimulate FGF-23 production by bone and FGF-23 targets the distal tubule to increase sodium reabsorption, and a FGF-23 soluble Klotho (sKl) regulatory axis, where FGF-23 suppresses α-Kl expression in the distal tubule leading to reduction in sKl and loss of the hormonal effects of this antiaging hormone. In this schema, FGF-23 regulates innate immune response through suppression of 1,25(OH)D2 production by the kidney. (B) Paracrine effects of FGF-23 on innate immune responses. Macrophages do not normally express FGF-23 or α-Klotho, but in the setting of infection, LPS/IFNγ induces the ectopic expression of both FGF-23 and its co-receptor α-Klotho that reconstitutes paracrine FGF-23 signaling in macrophages. FGF-23 stimulates pro-inflammatory responses in M1 macrophages and blocks the transition to M2 macrophages (3). In addition, FGF-23 is proposed to directly activate FGFR2 in PMNs to decrease recruitment (19).