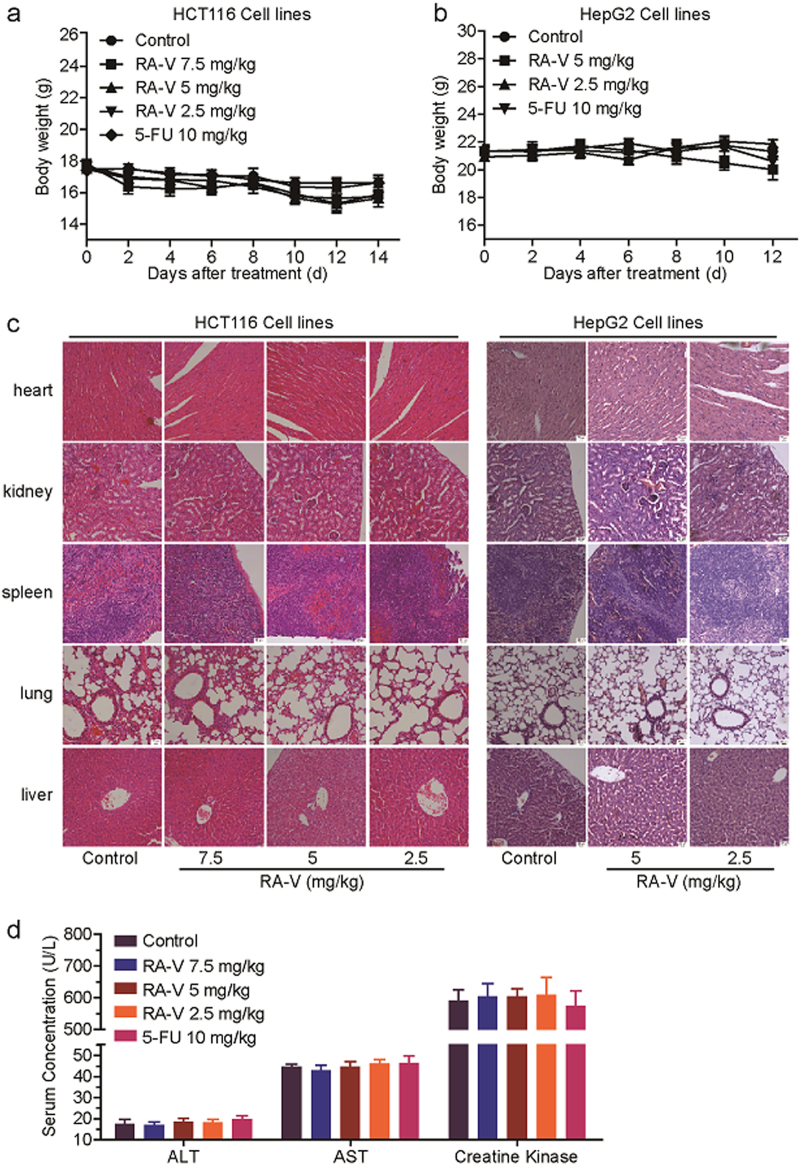
Fig. 8. Analysis of potential side effects for treatment of RA-V.
a–b The change curves of body weights of BALB/c bearing HCT116 (a) or HepG2 (b) xenograft tumors (n = 8 or 7). c Representative hematoxylin–eosin staining of heart, kidney, spleen, lung, and liver from vehicle- and various concentrations of RA-V-treated group. d The evaluation of serum ALT, AST, and creatine kinase for vehicle- and various concentrations of the RA-V-treated group