Fig. 1.
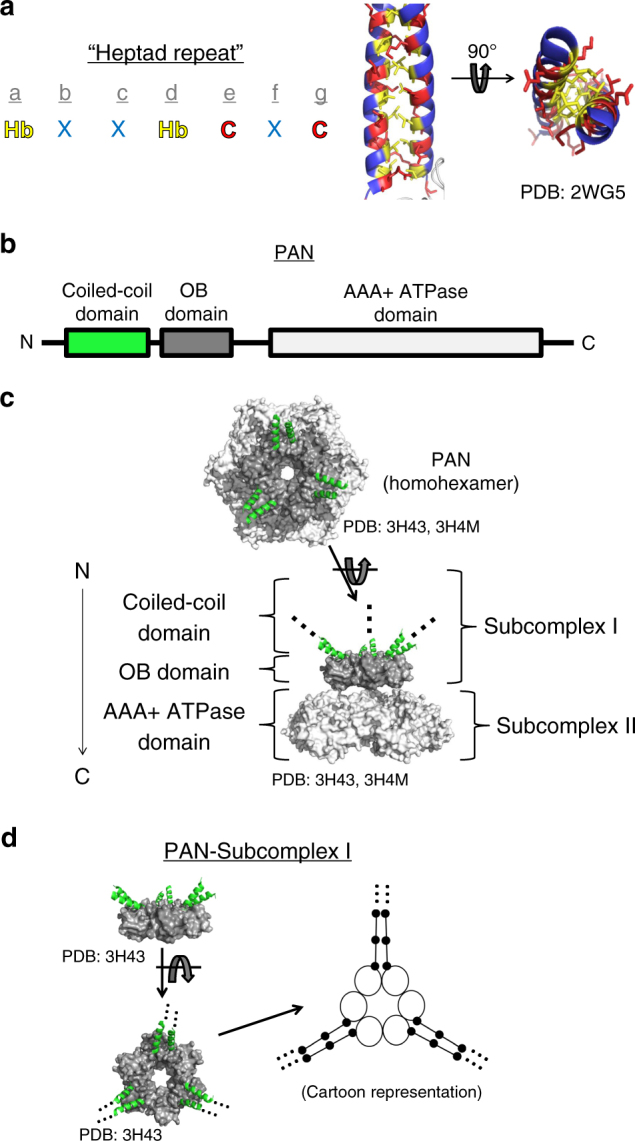
PAN contains three right-handed, dimeric coiled coils on its N-terminus. a Right-handed, dimeric coiled-coils (CCs) contain a seven-residue repeating consensus sequence (i.e., heptad repeat) where residues “a” and “d” are hydrophobic (Hb, yellow), “e” and “g” are charged (C, red), and the rest are typically polar (X, blue). Hb residues pack on the interior of the CC and form the main stabilizing interactions (PDB: 2WG5 [10.2210/pdb2WG5/pdb]). b The proteasome-activating nucleotidase (PAN) from archaea contains an N-terminal CC domain, followed by an oligonucleotide/oligosaccharide binding (OB) domain and a AAA+ ATPase domain. c Crystal structures of the N-terminus of PAN (subcomplex I, or CC-OB domain) show PAN’s six identical subunits in a symmetric ring (PDB: 3H43 [10.2210/pdb3H43/pdb]). The AAA+ ATPase domain (PDB: 3H4M [10.2210/pdb3H4M/pdb], light gray) also forms a hexameric ring underneath the OB domain (dark gray). Three dimeric CC domains protrude from the OB domains (green α-helices). The OB and ATPase domains domain are shown as a spacefill and the CC domains are shown as helices (PDB: 3H4M [10.2210/pdb3H4M/pdb], 3H43 [10.2210/pdb3H43/pdb]). d The structure of PAN’s CC-OB domain (subcomplex I) shown from a side and top view. An arrow points to the cartoon version of the CC-OB domain complex. The backbone of the CC domains are depicted as unwound sticks, while the black dots represent the inward facing hydrophobic residues. PDB: 3H43 [10.2210/pdb3H43/pdb]
