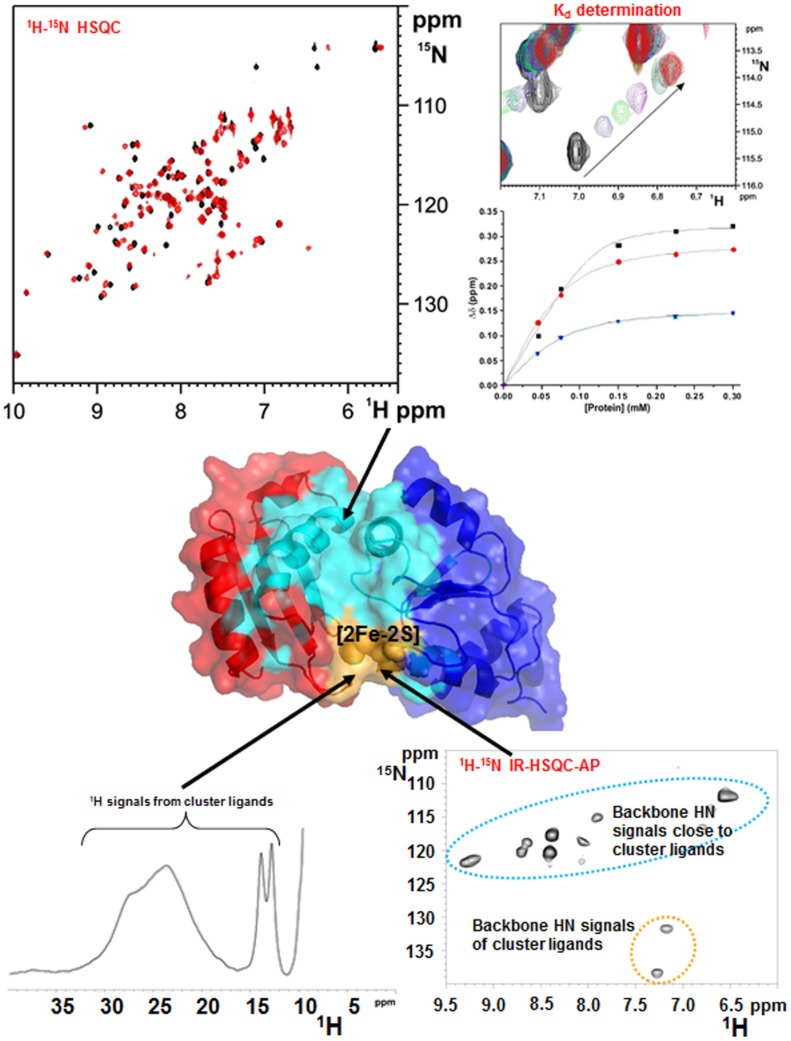
Fig. 4.
Solution NMR as a tool to investigate weak, transient protein–protein interactions in Fe–S protein maturation pathways. Weak and transient protein–protein interactions are detected by following backbone NH chemical shift changes occurring in a standard 1H–15N HSQC NMR experiment and in a 1H–15N IR-HSQC-AP NMR experiment, upon titrating 15N-labeled protein with the unlabeled protein partner and vice versa. Standard 1H–15N HSQC experiments allow the identification of protein–protein interacting regions far from the paramagnetic Fe–S cluster (showed in cyano), and to estimate the dissociation constant (Kd) of the observed interaction. 1H–15N IR-HSQC-AP NMR experiment allows to identify protein–protein interacting regions close to the paramagnetic Fe–S cluster (showed in yellow). 1D 1H NMR experiment provides information on the kind of Fe–S cluster(s) bound or assembled on a target protein or protein–protein complex, on the redox state(s) of the cluster(s), and on the cluster ligands