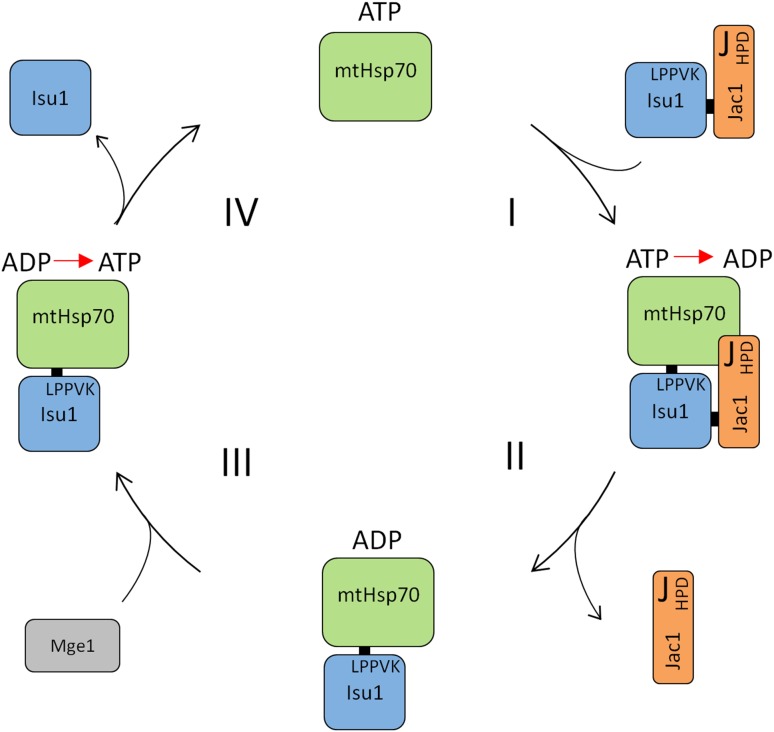
Fig. 2.
ATPase cycle of chaperone system involved in FeS biogenesis. J-type co-chaperone, Jac1, forms a complex with substrate, Isu1 and facilitate its delivery to mtHsp70. (I) The stability of the mtHsp70-protein substrate interaction depends on the conformation of the chaperone, which is regulated by the bound nucleotide. When ATP is bound, binding of substrate is relatively unstable. Therefore, ATP hydrolysis converts mtHsp70 to the form which has a relatively stable interaction with Isu1. Jac1 interacts directly with mtHsp70 and increases the stability of the mtHsp70-Isu1 interaction by stimulating the ATPase activity of mtHsp70 (II). Exchange of ADP for ATP results in dissociation of the bound Isu1. Exchange of ADP for ATP within mtHsp70 is supported through the cooperation with a nucleotide exchange factor (NEF). In yeast mitochondria, the only known NEF is Mge1 (III). Binding of ATP results in dissociation of the bound Isu1 and makes mtHsp70 ready for the next cycle (IV) [56]