Fig. 5.
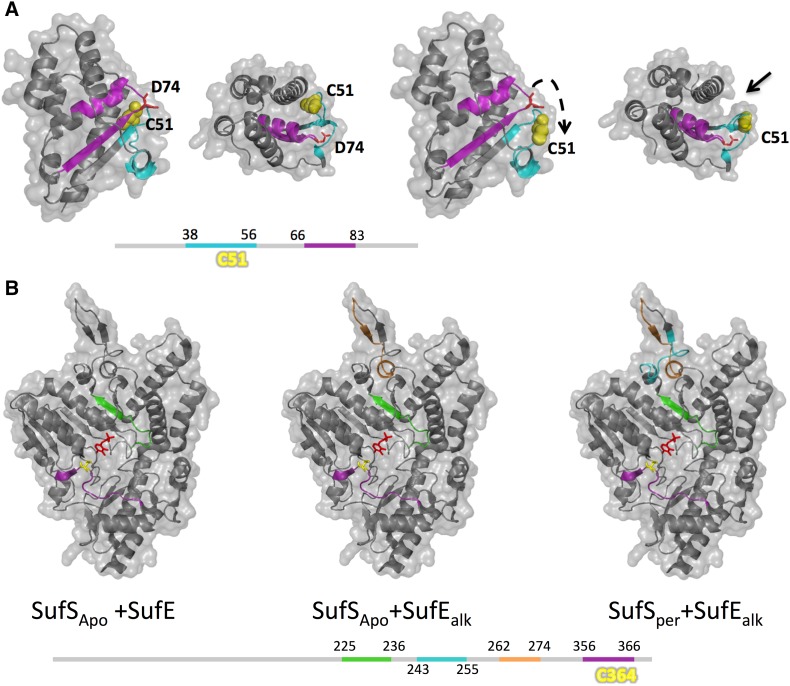
Interactions studies of SufS–SufE by HDX-MS. a Effect of SufS on SufE protein by HDX trapping assays. The model represents SufE protein before (left panels, two orientations) and after (right panels, two orientations) interaction with SufS. At the bottom of a is represented linear SufE sequence with important peptides whose accessibility to deuterium is modified by SufS interaction. The interaction between SufE and SufS implicated deuterium protection of peptide 38–56 (cyan) containing C51 and peptide 66–83 (magenta) of SufE. The C51 flipping process in the presence of SufS (represented by the black hatched arrow) leads to C51 solvent accessibility and the formation of a groove (black arrow) (manual representation by pymol). b Effect of SufE on SufS protein by HDX trapping assays. The model represents SufS protein. At the bottom of b is represented linear SufS sequence with important peptides whose accessibility to deuterium is modified by SufE interaction. The persulfide C364–SSH is indicated (in yellow) closed to the PLP cofactor labeled in red. Interaction between SufSApo and SufE implicated deuterium protection of peptide 225–236 (green) and 356–366 (magenta). Interaction between SufSApo and SufEalk implicated deuterium protection of peptides 225–236 (green), 262–274 (orange) and 356–366 (magenta). Interaction between SufSper and SufEalk implicated deuterium protection of peptides 225–236 (green), 262–274 (orange) and 356–366 (magenta) and increase accessibility of peptide 243–255 (cyan)