Fig. 7.
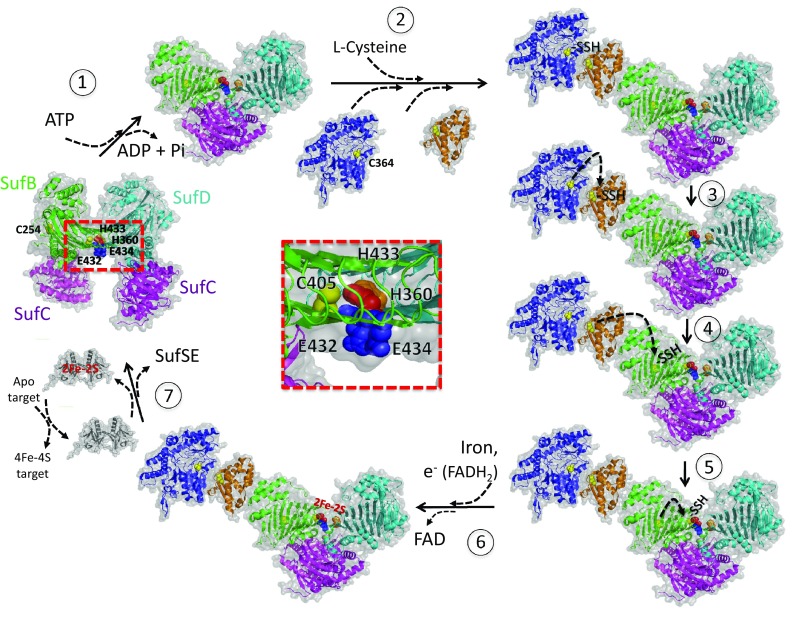
Current proposed mechanism for Fe–S assembly by the SUF system. SufBC2D complex is in a relaxation mode. (1) The mechanism is initiated by ATPase activity of SufC. Upon ATP binding, SufC transiently forms a dimer that elicits a significant conformational change of the entire SufBC2D complex. The SufB C405 and likely SufD H360 become exposed to the surface. This confirmation of the complex is favorable to recruit SufE–SufS complex. (2) Cysteine desulfurase activity (arrival of l-cysteine) generates a persulfide on SufS (C364) that is transferred to C51 of SufE (3) and then to C204 (4) and C405 (5) of SufB. (6) Arrival of iron and electrons (FADH2) allows building of Fe–S cluster on the complex at the SufB–SufD interface. SufD H360, SufB C405, E434 and H433 or E432 can be involved in Fe–S coordination or Fe–S formation. (7) SufSE release allows transfer of the SufBC2D cluster to SufA that can maturate 4Fe–4S target proteins. The original apo-SufBC2D complex is regenerated and ready for a new cycle. Critical amino acid are represented with bowls. Amino acids localized at the SufB–SufD interface (red square) are zoomed in the inset in the middle of the figure. SufU, not represented, is suggested to play a SufE-like role
