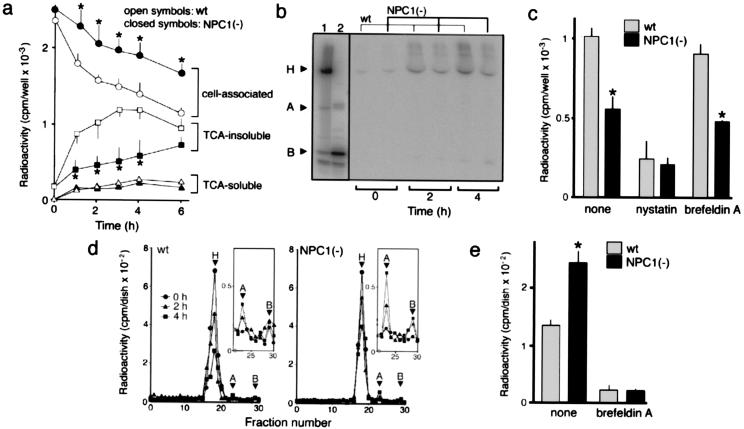
Figure 6.
Intracellular trafficking of 125I-CT. In all experiments, cells were incubated with 10 nM 125I-CT at 4°C for 1 h, washed, and further incubated in fresh medium at 37°C for the time indicated. Cells were on six-well plates in a–c, 60-mm dishes in d, and 100-mm dishes in e. (a) Time-dependent compartmentalization of radioactivity. Each fraction were recovered as described in Materials and Methods and counted for radioactivity. (b) SDS/PAGE analysis of radioactivity released into the medium. H, A, and B indicate the positions of the holotoxin, A and B monomers. Lane 1, 125I-CT applied; lane 2, 125I-CT after boiling in reducing SDS sample buffer containing 1 mM β-mercaptoethanol. (c) Effects of nystatin and brefeldin A on the radioactivity recovered in TCA-insoluble fractions of the medium. Cells were incubated at 37°C for 2 h. In both the 4°C and 37°C incubations, the medium contained nystatin (25 μg/ml) or brefeldin A (1 μg/ml). Neither drug caused a significant change in the cell-associated radioactivity at the end of the 4°C incubation (data not shown). (d) Gel filtration chromatography of cell-associated radioactivity. The positions of the holotoxin and A and B subunits were determined from elution profiles of 125I-CT before and after boiling in reducing SDS sample buffer (not shown). Profiles of fractions 22–30 depicted in an enlarged scale (Insets) show the amounts of A subunits. (e) Effects of brefeldin A on the release of A subunits. Cells were incubated at 37°C for 4 h with or without brefeldin A treatment as in c, and the intracellular amounts of A subunits were determined by gel filtration chromatography. All in a, c, and e, each point or bar represents the mean ± SEM (n = 3). *, P < 0.05, significantly different from the values of wt cells.