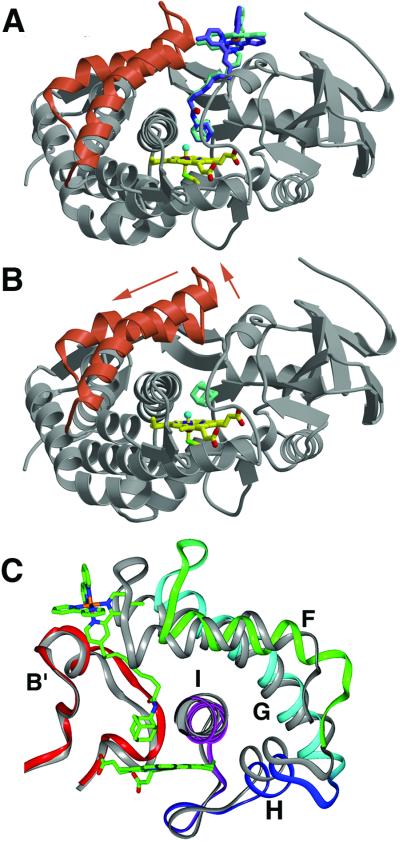
Figure 1.
Comparison of P450cam bound to Ru-C9-Ad (A) and adamantane (B) (16). On binding the Ru-substrate (Λ stereoisomer in blue, Δ stereoisomer in green) the F and G helices (red ribbons) retract from the P450cam β-sheet domain (gray ribbons). The adamantyl moiety binds in the same position above the heme (yellow) as free adamantane. (C) Movement of the F, G, H, and I helices (rotated ≈180° from A and B). For comparison, P450cam bound to camphor is shown in gray. Residues on the F/G loop move as much as 7.5 Å as the F and G helices slide approximately one helical turn (4.5 Å) across the I helix. The H helix (218) and the N terminus of the I helix (234) shift with the G helix to conserve interhelical contacts.