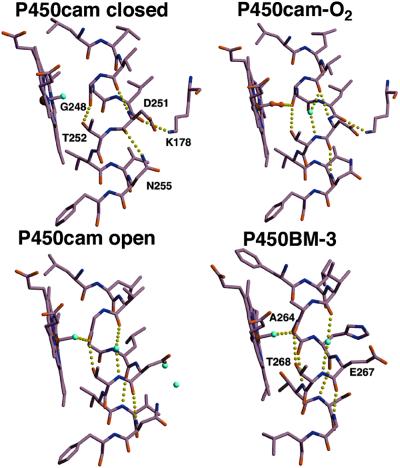
Figure 6.
The active sites of P450BM-3 and P450cam (closed, dioxygen-bound, and open conformations). Regularization of the I helix between Leu-250 and Asn-255 compensates for the loss of main-chain hydrogen bonds between Leu-245 and Leu-250 in the open P450cam structure. Interactions with the F and G helices break the hydrogen bond between the Asn-255 side-chain amide and the Asp-251 carbonyl, allowing the 251–252 peptide to flip down and hydrogen bond to the Asn-255 peptide amide. As in dioxygen-bound ferrous P450cam, this peptide flip is accompanied by the introduction of a helix-bridging water molecule (43). Movement of the F and G helices also breaks the hydrogen bond between Lys-178 and the peptide carbonyl of Leu-250, allowing the 250–251 peptide bond to flip 90° and anneal into the helix.