Figure 1. Structure-based design of an interdomain-locked HIV-1 Env trimer.
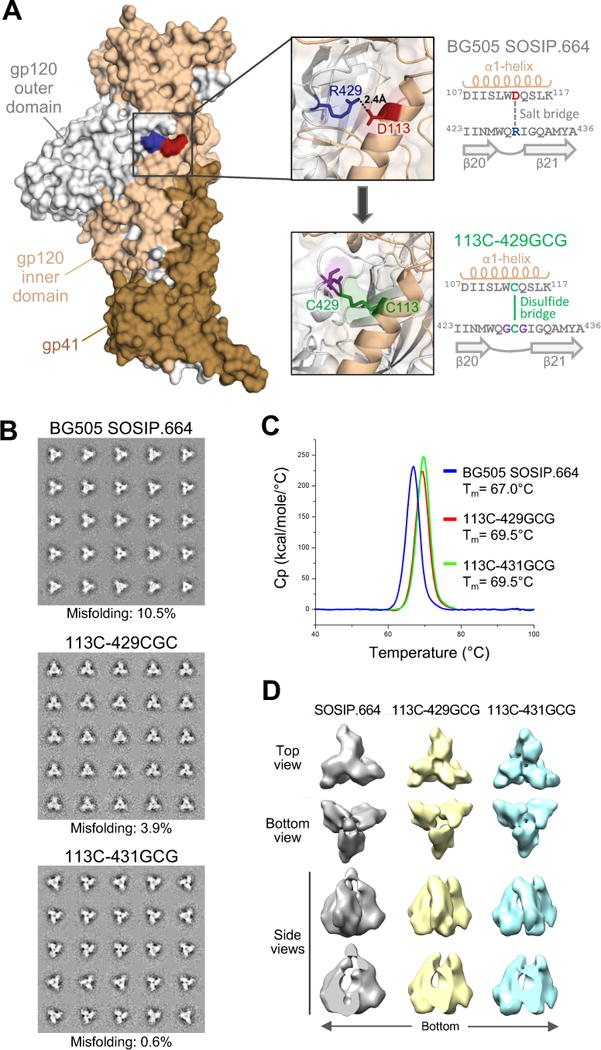
(A) Intramolecular interaction between the inner and outer domains of the gp120 Env glycoprotein. A single protomer from a crystal structure of the BG505 SOSIP.664 trimer (PDB ID: 4TVP) is shown in surface representation, highlighting the interdomain contact between R429 in the β20-21 loop (blue) and D113 in the α1-helix (red) of gp120. The upper inset shows a semi-transparent magnification of the interactive region with the side chains of R429 and D113 highlighted in stick representation, with the neighboring amino acid sequences reported in the scheme on the right. The design of an interdomain-locked mutant (D113C-R429GCG) bearing a neo-disulfide bridge (green) is illustrated in the lower inset, with the sequences reported in the scheme on the right.
(B) Negative-stain EM analysis of purified unmutated and interdomain-stabilized BG505 SOSIP.664 trimers. The 2D class averages are shown. The rate of misfolding was evaluated by analysis of loop movement, compactness and angles between individual protomers.
(C) Thermal stability of unmutated and interdomain-stabilized BG505 SOSIP.664 trimers as assessed by differential scanning calorimetry (DSC).
(D) Negative-staining electron microscopy (NS-EM) three-dimensional reconstructions of unmutated and interdomain-stabilized BG505 SOSIP.664 trimers. See also Figures S1, S2, S3 and S4, and Table S1.
