Abstract
Timing disturbances have being proposed as a key component of schizophrenia pathogenesis. However, the contribution of cognitive impairment to such disorders has not been clarified. Here, we investigated duration estimation and predictive timing in 30 patients with DSM-5 diagnosis of schizophrenia (SZ) compared to 30 healthy controls (HC). Duration estimation was examined in a temporal and colour discrimination task, fully controlled for working memory (WM) and attention requirements, and by more traditional temporal production and temporal bisection tasks. Predictive timing was measured in a temporal and spatial orienting of attention task. Expectations about stimulus onset (temporal condition) or location (spatial condition) were induced by valid and invalid symbolic cues. Results showed that discrimination of temporal and colour stimulus attributes was equally impaired in SZ. This, taken with the positive correlation between temporal bisection performance and neuropsychological measures of WM, indicates that duration estimation impairments in SZ are underpinned by WM dysfunction. Conversely, we found dissociation in temporal and spatial predictive ability in SZ. Unlike controls, patients were selectively unperturbed by events appearing at an unexpected moment in time, though were perturbed by targets appearing at an unexpected location. Moreover, patients were able to generate temporal expectations more implicitly, as their performance was influenced by the predictive nature of the flow of time itself.
Our findings shed new light on the debate over the specificity of timing distortions in SZ, providing evidence that predictive timing is a precise marker of SZ, more sensitive than duration estimation, serving as a valid heuristic for studying the pathophysiology of the disorder.
Keywords: Time perception, Predictive timing, Duration estimation, Working memory, Comorbid cognitive deficits, Temporal expectations
1. Introduction
Timing is a key component of cognition and the ability to process event duration and to integrate contextual information into a predictive framework plays a pervasive role in the continuity of consciousness (Fuchs, 2013). Indeed, disorders in which the constitution of reality is disturbed, like schizophrenia (SZ) and psychotic conditions are characterized by alterations in the experience of time. That's why temporality and its disturbances have been a major topic in psychopathology, and the transdisciplinary link between psychiatry and philosophy (Fuchs and Van Duppen, 2017).
Notably, since temporal cognition is a fundamental “basic unit of ability” on which other cognitive and behavioural processes are based (Allman and Meck, 2012), some models integrate time perception as a key component of schizophrenia pathogenesis (Andreasen et al., 1998; Fuchs and Van Duppen, 2017; Vogeley and Kupke, 2007). However, although the notion of mistimed information transfer in schizophrenia (i.e. cognitive dysmetria) -which is one of the most popular theories on the cognitive impairments and clinical outcomes in the disorder-, assumes that distorted temporal processing may underlie SZ symptoms (Andreasen et al., 1998), the existence of a genuine time perception deficit in the illness is still unproven (Roy et al., 2012). As a matter of fact, the significant correlation between time perception and cognitive performance in processes typically affected in SZ (Lee et al., 2009) casts doubt as to whether deficits in timing tasks reflect a pure temporal perception dysfunction or schizophrenia-related cognitive disturbances (Roy et al., 2012). Interpretation of the handful of studies investigating the impact of concurrent impairments in cognitive processes required for timing abilities -such as attention and working memory (WM)- (Bolbecker et al., 2014; Carroll et al., 2008; Elvevåg et al., 2004; Lee et al., 2009; Penney et al., 2006; Roy et al., 2012) is rather problematic as the neuropsychological tests used (e.g. Digit Span) likely tap different WM components than those required to estimate duration, thus impeding a definitive conclusion.
Likewise, current neurocognitive and computational theories propose that the positive symptoms of schizophrenia are due to an abnormality in the brain's inference mechanism, such that a failure in the integration and exchange between incoming sensory information and expectations is related to the emergence of delusions and hallucinations (Adams et al., 2013; Fletcher and Frith, 2009; Fuchs, 2007; Sterzer et al., 2016). Indeed, patients appear to have difficulty in predicting events over very short time intervals (Giersch et al., 2016; Lalanne et al., 2012) and in efficiently coding events in time suggesting that prediction impairments in patients may concern the prediction of time in particular (Giersch et al., 2015; Peterburs et al., 2013; Waters and Jablensky, 2009). Predictive timing disturbances in SZ have been mostly inferred from electrophysiological studies (Todd et al., 2003, Todd et al., 2008; Umbricht and Krljesb, 2005) although a recent behavioural investigation has established a link between predictive timing and clinical symptoms of SZ (Martin et al., 2017). Nevertheless, no significant difference in predictive timing performance between SZ and healthy controls was found in the abovementioned study.
Therefore, either level of explanation, one assuming that the fundamental problem underlying positive symptoms in SZ is the abnormal perception of time, or the other that dysfunctional predictive timing may cause both abnormal perceptions and abnormal beliefs, lack conclusive experimental evidence.
The present study has a threefold objective: (i) to investigate the extent of SZ's potential impairment in time estimation as a function of interval duration and task demands; (ii) to assess the contribution of comorbid WM and attention impairments on possibly reduced accuracy and precision in explicit duration estimation in SZ; (iii) to examine whether SZ patients have alterations in predictive coding, particularly in forming temporally specific expectations. Time perception performance was investigated using tasks for which the goal was to provide an overt estimate or representation of elapsed time (i.e. explicit timing: events duration discrimination, comparison of temporal stimuli to a reference, time intervals production) and a temporal and spatial orienting task in which predictive cues provided information on where (spatial condition) or when (temporal condition) a target will occur (implicit timing) (Piras and Coull, 2011).
2. Methods
2.1. Participants
An a priori power calculation in G*power was used to determine the minimum sample size (Supplementary Materials). Thirty patients with a DSM-5 (American Psychiatric Association, 2013) diagnosis of SZ were enrolled in the present study. Diagnosis of SZ was made by a senior psychiatrist and confirmed using the Structured Clinical Interview for DSM-5 Disorders- Clinician Version (First, 2016a). Inclusion criteria for all participants were: (1) age between 18 and 60 years, (2) at least 8 years of education, and (3) sufficient attentional resources to perform the experimental tasks (i.e. mean omission rates <20%). Full exclusion criteria are given in Supplementary Materials. Antipsychotic dosage was converted to chlorpromazine equivalents (CPZE); no patient was undertaking benzodiazepine-type drugs. Psychotic symptoms were rated using the Positive and Negative Syndrome scale -PANSS- (Kay et al., 1989).
Thirty healthy controls subjects (HC), age-sex matched, were recruited from the same geographical area. All HC were screened for current or past diagnosis of any DSM-5 Axis I or II disorder using the SCID-5 Research Version edition (First et al., 2015) and the SCID-5- Personality Disorders Interviews (First, 2016b). The same exclusion criteria for SZ patients were applied to HC.
All subjects underwent an attentional and WM cognitive evaluation which included: the Trail Making Test (TMT) (Reitan, 1992) and a non-verbal, non visuo-spatial working memory task, the Delayed Item Recognition task (DIR, see Supplemental Fig. 1) (Habeck et al., 2012) to measure the maintenance component of WM necessary for timing tasks. All participants gave written informed consent to participate after the procedures had been fully explained. The study was approved and carried out in accordance with the guidelines of the IRCCS Santa Lucia Foundation Ethics Committee.
2.2. Experimental procedure
Duration estimation was investigated with two perceptual timing tasks (i.e. temporal and colour discrimination task, temporal bisection task) and with a motor timing task (temporal production task). A temporal and spatial orienting of attention task assessed predictive timing. Detailed description of the temporal bisection and production tasks, analyses and results for the DIR task, the neuropsychological evaluation and the correlational analyses are included in Supplementary Materials.
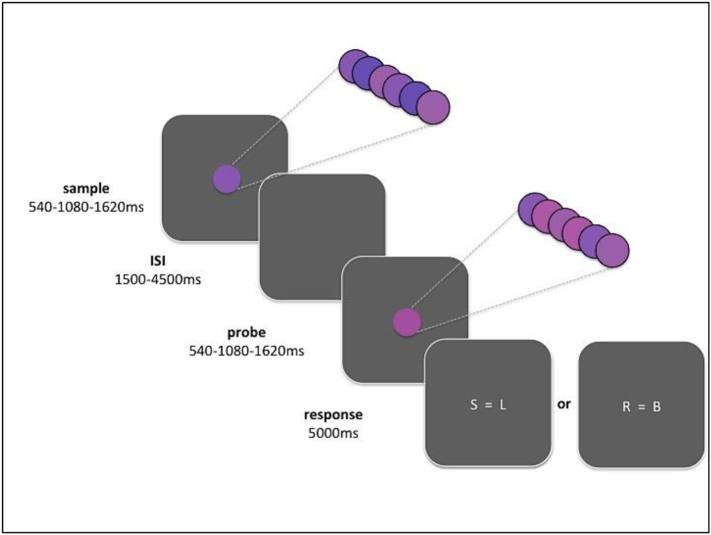
In the temporal and colour discrimination task (Coull, 2004; Coull et al., 2008, Coull et al., 2012) participants were asked to judge either the relative duration (shorter, equal to, longer) or the colour (redder, equal to, bluer) of two consecutive stimuli, by pressing one of three response buttons (Fig. 1). Stimulus colour was not uniform but it flickered rapidly. This colour manipulation was crucial for equating sustained attention and WM demands across the two conditions since, like perception of elapsed time, perception of colour required the integration of information throughout stimulus presentation. A response screen was presented for 5 s, and any slower response was not recorded.
Fig. 1.
Temporal and colour discrimination task. Each trial started with a random ITI of 1000–1900 ms. Consecutive sample and probe stimuli consisting in a central ball of one of three shades of purple (maroon, violet or indigo) were presented for one of three durations (540, 1080 or 1620 ms) after a pseudo-random inter-stimulus-interval (ISI) of 1500–4500 ms. Stimulus colour was not uniform but it flickered rapidly (90 ms), alternating presentations of five different shades of purple throughout stimulus duration, which provide the overall colour percept. Stimulus attributes were balanced across trails (18 each for 2 blocks) such that any of the three stimulus durations could be paired with any of the three colours. Participants were asked to estimate whether the second (probe) stimulus was shorter (S), equal (=) or longer (L) than the sample (time condition) or redder (R), equal (=) or bluer (B) than the sample (colour condition) by pressing one of three response buttons. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
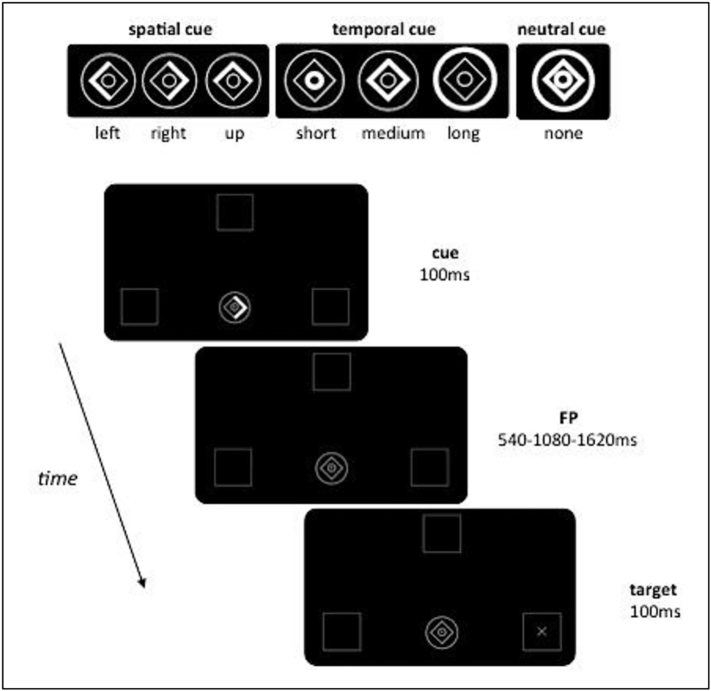
An adaptation of the temporal and spatial orienting of attention task (Coull and Nobre, 1998), measured reaction time (RT) to targets appearing after one of three intervals in one of three boxes depicted on the computer screen (Fig. 2). Subjects were asked to press a response button to detect the target, as quickly as possible, using information provided by one of three types of cue. In the majority of trials (80%) informative cues validly predicted where (spatial) or when (temporal cue) the target would appear (“valid” trials). In the remaining 20% of trials, the cue incorrectly predicted the spatial location or temporal onset of the target (“invalid” trials). In the neutral condition, no spatial or temporal information was provided. The three experimental conditions (spatial/temporal/neutral) were presented separately in three blocks of trials.
Fig. 2.
Temporal and spatial orienting task. Participants detected, as quickly as possible, briefly presented (100 ms) visual targets (“+” or an “x”) appearing at one of three locations (left/up/right) after one of three intervals (short/medium/long). Expectations about stimulus location (spatial condition) or onset (temporal condition) were conveyed by symbolic cues with 80% validity. The cue consisted of a central image (1° eccentricity) composed of a diamond and two rings. Part of the cue briefly brightened (100 ms) to inform participants on the possible spatial location or temporal onset of the upcoming target. During the spatial condition, the left, up or right side of the diamond was highlighted to inform subjects that the target was likely to appear in the left, up or right peripheral box. In the temporal condition, brightening of the inner circle, diamond or the outer circle indicated that the target would occur after a short (540 ms), medium (1080 ms) or long (1620 ms) delay, respectively. During the neutral condition, no spatial or temporal information was provided. Spatial locations and temporal onsets were balanced across trials for all the three experimental conditions. The inter-trial interval (ITI) varied randomly from 600 to 1000 ms. The three experimental conditions (spatial/temporal/neutral) were presented separately in three blocks of trials (90 each) and order of blocks counterbalanced across subjects.
2.3. Data analysis
Demographic data were compared using unpaired t-tests for age and educational attainment and chi-square test for gender.
2.3.1. Time and colour discrimination task
d-prime accuracy was computed for time and colour conditions separately, and analyzed in one repeated measures ANOVA with diagnostic group (SZ/HC) as between-subjects factor and stimulus attribute (time/colour) as within-subjects factor.
2.3.2. Temporal bisection task
Individual mean proportion of longer than sample p(long) responses was plotted as a function of each probe duration (699, 816, 933, 966, 1033, 1066, 1183, 1299 ms), and analyzed in one 2 × 8 repeated measures ANOVA with diagnostic group (SZ/HC) as between-subjects factor and duration as within-subjects factor. Psychometric curves were fitted to individual subjects' data and two measures of temporal sensitivity calculated: the bisection point (i.e. Point of Subjective Equality -PSE-, a measure of temporal sensitivity), and d-prime (Green and Swets, 1966) a general index of perceptual discrimination accuracy. PSE and d-prime were analyzed as dependent variables in two factorial one-way ANOVAs, with group (SZ/HC) as independent variable.
2.3.3. Temporal production task
Reaction times (RTs) faster than 200 ms (i.e. omissions: SZ = 5%; HC = 1%) were not included in the analyses. Individual mean RTs and standard deviation were analyzed as dependent variables in two factorial one-way ANOVAs, with diagnostic group (SZ/HC) as independent variable. Individual press rates were plotted as a function of the interval between the tone onset and the subject's motor response (parcelled into seven 200 ms time bins, range: 200–1600 ms), and analyzed in one 2 × 7 repeated measures ANOVA with group (SZ/HC) as between-subjects factor and time-bin as within-subjects factor. Individual subjects' data were fit on a Gaussian curve and the distribution peak (assumed to represent the subject's internal representation of one second duration) analyzed as dependent variable in one factorial one-way ANOVA, with group (SZ/HC) as independent variable.
2.3.4. Temporal and spatial orienting of attention task
RTs to target stimuli faster than 100 ms (SZ = 8%; HC = 3%) were considered anticipatory and removed from the analysis. Mean RTs were then analyzed in one repeated measures ANOVA with diagnostic group (SZ/HC) as the between-subjects factor and cue type (temporal/spatial), validity (valid/invalid) and foreperiod (FP, i.e. the interval between a warning signal and the target: 540 ms/1080 ms/1620 ms) as within-subjects factors. To better explore the potential benefits of temporally valid (TV) cues and costs of temporally invalid (TI) cues on performance, one repeated measures ANOVA, with group (SZ/HC) as between-subjects factor and cue type (neutral/TV/TI) and FP (540 ms/1080 ms/1620 ms) as within subjects factors was executed. The same analysis was performed for the spatial condition. In order to ascertain whether RTs slopes were comparable in the two groups across the entire distribution of RT latencies (10th, 25th, 50th, 75th, and 90th percentiles) four 2 × 5 repeated measures ANOVAs with diagnostic group (SZ/HC) as between-subjects factor, and percentile as within-subjects variable were performed separately for cue type (temporal/spatial) and validity (valid/invalid). Lastly, to explore FP effects unconfounded by cueing, a separate repeated measures ANOVA was performed for the neutral condition, with FP (current FPn/previous FPn-1) and duration (540 ms/1080 ms/1620 ms) as within-subjects factors and group (SZ/HC) as the between-subjects variable.
2.3.5. Correlational analysis
Aside from clinical variables (CPZE, duration of illness, number of hospitalizations, five-factors PANSS scores) only timing and cognitive measures that significantly differed between SZ and HC (p < 0.05) were included in stepwise regression analyses (Supplementary Material).
3. Results
The two groups did not significantly differ in terms of age and gender, while educational attainment was significantly higher in the HC group (Table 1).
Table 1.
Sociodemographic and clinical characteristics of 30 SZ and 30 HC.
| SZ (N = 30) Mean (SD) | HC (N = 30) Mean (SD) | t or χ2 | df | p | |
|---|---|---|---|---|---|
| Age (years) | 41.36 (9.87) | 39.6 (10.93) | −0.66 | 58 | ns |
| Educational Level | 12.7 (2.89) | 15.83 (2.84) | 4.23 | 58 | <0.0001 |
| Gender | 21 M/9F | 19M/11F | 0 | 1 | ns |
| Equivalents of chlorpromazine | 292.23 (208.37) | – | – | – | – |
| Duration of illness (years) | 17.53 (8.64) | – | – | – | – |
| PANSS POS | 15.86 (6.55) | – | – | – | – |
| PANSS NEG | 21.03 (6.66) | – | – | – | – |
| PANSS cognitive | 10.13 (3.98) | – | – | – | – |
| PANSS hostility | 6.26 (1.87) | – | – | – | – |
| PANSS emotional discomfort | 7.9 (3.9) | – | – | – | – |
3.1. Time and colour discrimination task
Performance was significantly different across task dimensions [F(1,1) = 7.286; p = 0.009], indicating that participants performed the temporal task better than the colour one. Despite a main effect of group, with SZ showing a significantly lower discrimination ability than HC [F(1,58) = 41.503; p < 0.0001], no interaction between group and dimension was found [F(1,58) = 1.624; p = 0.207] (Fig. 3).
Fig. 3.
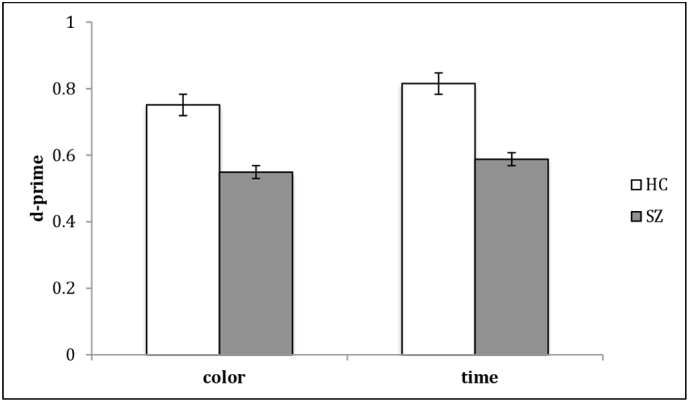
Time and colour discrimination -Results-. Mean accuracy for time and colour conditions separately. SZ patients were less accurate than controls in discriminating both temporal and colour stimuli characteristics.
3.2. Temporal bisection and production performance
3.2.1. Temporal bisection
Results from the ANOVA comparing SZ and HC mean proportion of p(long) responses showed no main effect of group [F(1,58) = 3.464; p = 0.121], but a main effect of duration [F(1,7) = 167.837; p < 0.001], and a significant group x duration interaction [F(7,406) = 5.947; p < 0.001]. Post hoc analysis indicated a significant difference in the proportion of p(long) responses at the shortest probe duration (699 ms [t(58) = −2.252; p = 0.028]), with SZ more likely to categorize this as a “long” duration than HC, and at the four longest ones (1033 ms [t(58) = 2.259; p = 0.014]; 1066 ms [t(58) = 2.294; p = 0.025]; 1183 ms [t(58) = 2.705; p = 0.008]; 1299 ms [t(58) = 3,29; p = 0.001]), with SZ less likely to categorize them as “long” (Supplementary Fig. 2). In contrast, the PSE calculated from SZ plotted data was not significantly different from that of HC [F(1,58) = 1.276; p = 0.263]. Results from the ANOVA comparing temporal bisection d-prime confirmed a general impairment in perceptual timing ability in SZ, such that patients showed a highly significant reduction in temporal discrimination sensitivity [F(1,58) = 15.384; p = 0.0002].
3.2.2. Temporal production
Results from the ANOVA comparing SZ and HC mean press rates as a function of time-bins showed significant main effects of group [F(1,58) = 6.13; p = 0.016] and time-bin [F(1,6) = 14.68; p < 0.001], and a significant interaction between the two [F(6,348) = 3.53; p = 0.002]. Post hoc analysis indicated that SZ were more variable than HC in estimating one-second duration (Supplemental Fig. 3). Patients were less likely to produce a 1000 ms response and more likely to produce very short or very long responses (400 ms bin [t(58) = −2.467; p = 0.016]; 1000 ms bin [t(58) = 2.181; p = 0.033]; 1200 ms bin [t(58) = 2.311; p = 0.024]; 1600 ms bin [t(58) = −2.409; p = 0.019]). This observation is supported by a significantly higher RTs standard deviation in patients compared to controls [F(1,58) = 6.295; p = 0.014]. By contrast, there were no significant group differences in mean RTs [F(1,58) = 2.348; p = 0.131] or in the peak of the curves calculated on individual subject data [F(1,58) = 1.896; p = 0.173], indicating that the RTs distribution was centred on the 1000 ms time bin in both groups.
3.3. Temporal and spatial orienting of attention task
A significant main effect of group [F(1,58) = 22.723; p < 0.0001] indicated that SZ were slower than HC in detecting targets, while a significant validity effect [F(1,1) = 107.466; p < 0.0001] confirmed the expected behavioural optimization, with faster RTs for targets appearing in a predictable location or moment in time (as opposed to spatially and temporally invalidly cued targets). While no significant interaction between group and validity was found [F(1,58) = 0.089; p = 0.767], the interaction between group, cue type and validity was significant [F(1,58) = 3.891; p = 0.053]. Post hoc analysis with paired t-tests showed dissociation in temporal and spatial invalidity effects across groups. HC were perturbed by invalid events (compared to validly cued) in both spatial [t(29) = −8.172; p < 0.0001] and temporal [t(29) = −3.71; p = 0.0009] dimensions, while SZ were perturbed by spatially invalid targets [t(29) = −7.451; p < 0.0001], but were unaffected by temporally unexpected ones [t(29) = −0.197; p = 0.845]. A significant main effect of FP [F(1,2) = 121.316; p < 0.0001] was qualified by a significant FP x validity interaction [F(1,2) = 9.725; p < 0.0001] indicating that performance was particularly disrupted for invalidly cued targets occurring earlier, instead of later than expected. However, group did not interact with FP [F(1,2) = 0.045; p = 0.956] and there was no significant group x FP duration x cue type x validity interaction [F(2,116) = 0.472; p = 0.625].
Another repeated measures ANOVA comparing neutral trials to TV and TI trials showed significant cue type x FP interaction [F(1,4) = 7.758; p < 0.0001] and significant group x cue type x FP interaction [F(1,4) = 3.154; p = 0.015]. In particular, post hoc analysis with paired t-tests revealed that, while HC showed significantly slower RTs for TI trials at the shortest FP compared to neutral ones [t(29) = −4.153; p = 0.002], SZ patients showed no such difference [t(29) = −0.996; p = 0.327]. By contrast, the same analysis performed on the spatial dimension showed a main effect of cue type [F(1,2) = 83.596; p < 0.0001], indicating slower RTs for spatially invalid cues compared to neutral ones, although the interaction with diagnostic group was not significant [F(1,2) = 2.368; p = 0.098], nor was the group x FP x cue type interaction [F(1,4) = 0.475; p = 0.754]. These results thus confirm that SZ patients have a specific problem with the invalidity effect selectively in the temporal, and not in the spatial, domain (Table 2).
Table 2.
Temporal and spatial orienting effects: mean reaction times (M) and standard deviation (SD) to uncued and cued target stimuli as a function of validity and foreperiod duration.
| Cue type |
HC |
SZ |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Neutral | ||||
| Short | 347.44 | 50.21 | 429.95 | 88.87 |
| Medium | 317.79 | 51.95 | 383.89 | 70.91 |
| Long | 302.31 | 45.4 | 369.25 | 63.57 |
| Temporal valid | ||||
| Short | 331.3 | 65.21 | 430.25 | 84.74 |
| Medium | 317.38 | 49.97 | 396.51 | 90.93 |
| Long | 301.22 | 46.98 | 381.83 | 86.47 |
| Temporal invalid | ||||
| Short | 376.22 | 66.87 | 438.91 | 101,64 |
| Medium | 306.97 | 52.28 | 386.86 | 94.71 |
| Long | 302.86 | 48.45 | 385.83 | 87.38 |
| Spatial valid | ||||
| Short | 346.3 | 64.38 | 432.32 | 100.00 |
| Medium | 307.06 | 48.89 | 384.94 | 95.07 |
| Long | 291.33 | 43.41 | 371.88 | 77.44 |
| Spatial invalid | ||||
| short | 422.28 | 92.53 | 514.19 | 113.64 |
| medium | 360.38 | 81.64 | 458.2 | 122.5 |
| long | 336.56 | 61.15 | 436.88 | 109.34 |
The reported effects were not imputable to a generic slowing down of SZ performance since validity equally affected slow and fast RTs in both groups and in both conditions (temporal/spatial) and SZ's RTs were not significantly different from HC's RTs across the entire distribution, as revealed by post hoc analyses on the interaction group x percentile in the TV, TI, spatial valid, and spatial invalid condition (from the 10th to the 90th percentile: p > 0.05).
When FP effects were examined in the neutral condition, we found a main effect of group (F(1,58) = 20.165; p < 0.001), a significant effect of foreperiod (F(1,58) = 8.21; p = 0.005) and duration (F(2,116) = 35.45; p < 0.001), and a significant foreperiod x duration interaction (F(2,116) = 123.56; p < 0.001), while no third level interaction with group was found (F(2,116) = 2.756; p > 0.05). Performance was influenced by FP duration in both SZ and HC (p < 0.001, i.e. classic foreperiod effect) and RTs were slower after longer, as opposed to shorter FPn-1 in both groups (p < 0.001, i.e. basic sequential effect) (Supplementary Table 1).
3.4. Correlation results
A significant positive correlation between patients' d-prime in the temporal bisection task and total mean accuracy in the DIR task (F(1,28) = 7.793; r = 0.5; adjusted R2 = 0.2; p = 0.009) was found. Temporal discrimination d-prime was positively correlated with colour discrimination d-prime (F(1,28) = 6.728; r = 0.4; adjusted R2 = 0.2; p = 0.014).
Three significant correlations were also found between timing variables that differed significantly between SZ and HC and clinical characteristics of the SZ group (see Supplementary Materials).
4. Discussion
To our knowledge, this is the first study that investigated timing abilities in SZ patients using a comprehensive experimental battery purposely designed to identify whether impaired timing is a core deficit in SZ or related to other cognitive dysfunctions.
The main finding here is that time perception performance (in the sense of explicit judgments of events duration or the production of time intervals) is strongly associated in schizophrenia, to well-known disorder-related cognitive impairments (Roy et al., 2012). Indeed, by controlling for the attentional and mnemonic components of the timing task (Coull et al., 2011, Coull et al., 2012), we found that patients were less accurate than controls in discerning both temporal and non-temporal stimulus attributes, implying that discrimination behaviour was disturbed generally in SZ. We also demonstrated that SZ patients were more variable than controls in estimating a one-second interval in a motor timing task, and exhibited widespread difficulty in categorising durations in a temporal bisection one. The overall change in the shape of performance distributions advocates that a generalised decrease in temporal sensitivity, rather than a shift in one direction or another, may account for SZ disturbances in duration estimation (Roy et al., 2012; Thoenes and Oberfeld, 2017). Additionally, temporal sensitivity in the bisection task was correlated to WM accuracy, but not to measures of attention and psychomotor speed, thus reinforcing our observation that timing deficits in SZ, at least those measured by this classic perceptual task, are a side-effect of a more fundamental WM impairment (Lee et al., 2009; Roy et al., 2012). The specific correlation with accuracy in a task measuring the maintenance component of WM suggests that perceptual timing performance in SZ is better explained, in the bisection task, by alterations in the encoding and maintenance component cognitive processes of duration estimation, rather than by deficits in timing per se. Indeed, a general deficit in discrimination behaviour could account for differences in performance in the time discrimination task (since SZ showed significantly lower discrimination ability also in the colour control task), while difficulties in relying on informative temporal cues and not in implicitly forming temporal expectations, could explain the observed differences in the temporal orienting of attention task. All the above considered, duration estimation impairments in SZ seem to be underpinned by deficits in concurrent cognitive processes and not consequent to a genuine time perception deficit.
Moreover, temporal variability in the production task increased linearly with both duration of illness and age of SZ patients thus suggesting that dysfunctions in a fronto-thalamo-striatal control network (Andreasen et al., 1998; Banaj et al., 2018), presumably hypo-activated (Alústiza et al., 2016) or disorganized already at early stages of the illness (Alústiza et al., 2016; Lambe et al., 2006), might mediate the observed variability, as it is involved in both time processing and the cognitive resources required for timing tasks (Radua et al., 2014).
However, previous developmental studies have shown that while explicit duration judgements are linked to WM capacity (Zelanti and Droit-Volet, 2011), more implicit measures of timing ability, such as reaction time tasks, are not (Droit-Volet and Coull, 2016). When patients' performance on a spatial and temporal orienting of attention task was compared to that of HC subjects we found that in normal controls RTs were slower when targets appeared in an unexpected location or moment in time, thus confirming the behavioural costs of invalid cueing in both spatial and temporal domain (Coull and Nobre, 1998). Conversely, while SZ patients were similarly perturbed by targets appearing at an unexpected location, their performance was unaffected when targets appeared earlier than expected, indicating a dissociation in temporal and spatial invalidity effects. It might be argued that temporal and spatial cueing effects are hardly comparable, as spatial arrow cues elicit both voluntary and involuntary attentional shifts (Hommel et al., 2001; Olk, 2014), being over-learned symbols of direction (Ristic and Kingstone, 2012) as opposed to temporal ones. As a matter of fact, SZ patients did appear to be implicitly forming temporal expectations, since in the uninformative neutral condition they showed the expected “variable foreperiod effect” (faster RTs as a function of the increasing conditional probability over time that the target will occur) (Niemi and Näätänen, 1981), as well as the classic “sequential effect” (faster RTs when the foreperiod of the previous trial is of the same duration or shorter than the current foreperiod) (Los et al., 2014). Moreover, when we assessed the relative cost of invalid versus neutral cues, we found that patients, unlike controls, did not show longer RTs (due to the fact that subjects are prepared for a longer interval and are “caught off guard”) when targets appeared unexpectedly earlier. No difference with controls was observed when targets appeared later than expected, thus indicating that SZ patients made better use of temporal expectations derived implicitly from the flow of time itself (“hazard function”(Luce and Robert, 1986)), than those that were explicitly provided by temporal cues.
Computational approaches that have conceptualized predictive processing as a Bayesian inference, suggest that disturbances in the integration and exchange between incoming sensory information and expectations may explain SZ pathophysiology (Roa Romero et al., 2016; Sterzer et al., 2016). This interpretation fits well with the observed dissociation in the behavioural advantage derived from implicitly versus explicitly formed temporal expectations, since patients relied more on sensory data (e.g. the evidence that an expected target will occur at some time in the future, given that it has not yet occurred) than on informative cues. Indeed, SZ seemed unable to combine, in the time domain, the acquired knowledge about the predictive value of informative signals with the sensory evidence, thus probably coming to the formation of false inferences (Adams et al., 2013; Fletcher and Frith, 2009). This is in line with experimental evidence demonstrating that not all predictive coding mechanisms are impaired in schizophrenia (Martin et al., 2017; Tizard and Venables, 1956; Zahn et al., 1963) while, at the same time, patients seemed to overcompensate the uncertainty induced by invalidly cued targets. Since predictive models are updated whenever predictions are violated by sensory data (Sterzer et al., 2016), it might be the case that the mere presence of temporally unexpected trials may have discouraged patients from forming expectations based on cue validity, leading them to rely solely on the temporal predictability conveyed by the hazard function. Such inadequate reliance on predictive temporal cues may be a consequence of unrealistic overconfidence in their judgments (self-certainty, Beck, 2004) and reluctance to consider corrective feedback (self-reflectiveness, Beck, 2004), typically shown by SZ patients (Orfei et al., 2010, Orfei et al., 2017; Spalletta et al., 2014). Since the time (as opposed to the spatial) domain allows beliefs about temporal predictability to be derived otherwise, patients might have been disinclined to form expectations according to the predictive nature of informative cues and in using them to update their beliefs after having seen the data (Adams et al., 2013).
Intriguingly, we also found a significant correlation between the attenuated behavioral cost of being invalidly cued and antipsychotic medication in SZ, such that low dosages were related to less reliance on predictive temporal cues. Given the dopaminergic hypothesis of schizophrenia (van Rossum, 1966) and the dopamine modulating effect of antipsychotics, the reported association might be driven by an insufficient regulation, at low antipsychotic dosages, of the predictive process leading to the formation of balanced expectancies (Adams et al., 2013). Indeed, prediction-error-related behaviours are sensitive to dopaminergic perturbations (Pessiglione et al., 2006) and an ineffectively treated hyper-dopaminergic state may have determined the observed overcompensation for uncertainty.
Moreover, the attenuated behavioural cost of invalid cueing was correlated with illness duration (though not age), such that patients characterized by a longer history of illness were more impaired in their use of temporally predictive cues. Since early onset schizophrenia patients show a poorer response to neuroleptic treatment (Vyas and Gogtay, 2012), the aberrant salience assigned to temporally unexpected trials may be related, once again, to a suboptimally controlled hyper-dopaminergic state (Kapur and Shitij, 2003).
Before concluding, some limitations of the present study should be addressed. First, the relatively small sample size might have weakened the robustness of findings. Nevertheless, our sample size is comparable to previous investigations of timing deficits in SZ (Carroll et al., 2009; Davalos et al., 2011; Turgeon et al., 2012). A second point is that although we adopted quite conservative patient inclusion criteria, we cannot exclude the possibility that clinical heterogeneity might have affected the present results. Duration of illness may be one such factor, as we found significative correlations between SZ onset and the temporal invalidity effect. Moreover, since disturbances in timing are related more to positive than negative symptoms, the predominance of negative symptoms and cognitive dysfunction in our sample may be another one. Future studies in which SZ patients are classified according to symptom dimensions and clinical characteristics, such as positive/negative symptoms and/or duration of illness, will help clarify the issue of possible patterns of interaction between timing deficit and clinical variables.
In conclusion, we demonstrated a selective deficit in using temporal, but not spatial, predictive cues to anticpate target occurrence in SZ patients, suggesting that predictive timing is a precise marker of the illness, more sensitive than duration estimation. Indeed, impaired duration estimation in the milliseconds-to-second range correlated with WM capacity and age, which calls into question whether the observed deficit was due to defective timing or to a more general WM impairment or cognitive decline. Moreover, patients' performance was equally impaired on a colour discrimination task, indicating a general non-selective WM deficit. Our findings shed new light on the debate over the specificity of timing distortions in SZ, providing evidence that predictive timing is an effective heuristic for studying the pathophysiology of schizophrenia.
Contributors
Valentina Ciullo, Federica Piras and Jennifer Coull designed the study, wrote the protocol, performed analyses and wrote the first draft of the paper. Daniela Vecchio and Nerisa Banaj managed literature search, collected data and contributed to behavioural analyses. Gianfranco Spalletta supervised the study design, behavioural analyses and results interpretation. All authors contributed to and have approved the final manuscript.
Funding body
This study was conducted within the project “Multidimensional study of timing abilities and sense of agency in schizophrenia and bipolar patients” founded through 5Xmille 2016 from the Italian Ministry of Health.
Conflict of interest
All the authors declare they have no conflicts of interest.
Acknowledgments
The authors would like to thank Dr.Fabrizio Piras for helping in results interpretation and the Mental Health Department Roma E for referring patients to be enrolled in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2018.04.001.
Appendix A. Supplementary data
Supplementary Fig. 1 The Delayed Item Recognition task. Participants had to hold in memory 1, 2 or 3 unnameable shapes for a maintenance interval (4 or 7 s) and to indicate whether or not the probe matched a shape included in the sample array using a two-choice button press at the onset of the response screen. The first level consisted of two blocks of 8 trials each and a fixed 7 s maintenance interval, while for the second and the third level four blocks of 8 trials each, with a fixed 7 s maintenance interval, were included (tot = 80 trials). Stimuli were selected pseudo-randomly from a pool of 400 shapes such that the same shape couldn't be presented twice. Percentage of probe-equal-to-sample and probe-different-from-sample trials was balanced across the experimental blocks, and the inter-stimulus interval was a fixed 3 s period of black screen.
Supplementary Fig.2 Temporal bisection, perceptual timing task -Results-. Individual mean proportion of longer than sample responses as a function of probe duration for HC and SZ.
Supplementary Fig.3 Temporal production, motor timing -Results-. Individual mean proportion of responses as a function of 200 ms time bins (range: 200-1600 ms) for HC and SZ.
References
- Adams R.A., Stephan K.E., Brown H.R., Frith C.D., Friston K.J. The computational anatomy of psychosis. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman M.J., Meck W.H. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alústiza I., Radua J., Albajes-Eizagirre A., Domínguez M., Aubá E., Ortuño F. Meta-analysis of functional neuroimaging and cognitive control studies in schizophrenia: preliminary elucidation of a Core dysfunctional timing network. Front. Psychol. 2016;7 doi: 10.3389/fpsyg.2016.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association DSM 5. Am. J. Psychiatry. 2013 [Google Scholar]
- Andreasen N.C., Paradiso S., O'Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Banaj N., Piras F., Piras F., Ciullo V., Iorio M., Battaglia C.…Spalletta G. Cognitive and psychopathology correlates of brain white/grey matter structure in severely psychotic schizophrenic inpatients. Schizophr. Res. Cogn. 2018;12:29–36. doi: 10.1016/j.scog.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. A new instrument for measuring insight: the Beck cognitive insight scale. Schizophr. Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Bolbecker A.R., Westfall D.R., Howell J.M., Lackner R.J., Carroll C.A., O'Donnell B.F., Hetrick W.P. Increased timing variability in schizophrenia and bipolar disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.A., Boggs J., O'Donnell B.F., Shekhar A., Hetrick W.P. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.A., O'Donnell B.F., Shekhar A., Hetrick W.P. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.T. Functional anatomy of the attentional modulation of time estimation. Science. 2004;80(303):1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Coull J.T., Nobre A.C. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. https://doi.org/0270-6474/98/187426-10$05.00/0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.T., Nazarian B., Vidal F. Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. J. Cogn. Neurosci. 2008;20:2185–2197. doi: 10.1162/jocn.2008.20153. [DOI] [PubMed] [Google Scholar]
- Coull J.T., Morgan H., Cambridge V.C., Moore J.W., Giorlando F., Adapa R., Corlett P.R., Fletcher P.C. Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology. 2011;218:543–556. doi: 10.1007/s00213-011-2346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.T., Hwang H.J., Leyton M., Dagher A. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J. Neurosci. 2012;32:16704–16715. doi: 10.1523/JNEUROSCI.1258-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D.B., Rojas D.C., Tregellas J.R. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophr. Res. 2011;127:123–130. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S., Coull J.T. Distinct developmental trajectories for explicit and implicit timing. J. Exp. Child Psychol. 2016;150:141–154. doi: 10.1016/j.jecp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Elvevåg B., Brown G.D.A., McCormack T., Vousden J.I., Goldberg T.E. Identification of tone duration, line length, and letter position: an experimental approach to timing and working memory deficits in schizophrenia. J. Abnorm. Psychol. 2004;113:509–521. doi: 10.1037/0021-843X.113.4.509. [DOI] [PubMed] [Google Scholar]
- First M.B. American Psychiatric Association; 2016. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders, Clinician Version. [Google Scholar]
- First M.B. American Psychiatric Association; 2016. SCID-5-PD: Structured Clinical Interview for DSM-5 Personality Disorders: Includes the Self-report Screener Structured Clinical Interview for DSM-5 Screening Personality Questionnaire (SCID-5-SPQ) [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association; 2015. Structured Clinical Interview for DSM-5 ® -Research Version. [Google Scholar]
- Fletcher P.C., Frith C.D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Fuchs T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology. 2007;40:229–235. doi: 10.1159/000101365. [DOI] [PubMed] [Google Scholar]
- Fuchs T. Temporality and psychopathology. Phenomenol. Cogn. Sci. 2013;12:75–104. [Google Scholar]
- Fuchs T., Van Duppen Z. Time and events: on the phenomenology of temporal experience in schizophrenia (ancillary article to EAWE domain 2) Psychopathology. 2017;50:68–74. doi: 10.1159/000452768. [DOI] [PubMed] [Google Scholar]
- Giersch A., Poncelet P.E., Capa R.L., Martin B., Duval C.Z., Curzietti M., Hoonacker M., van Assche M., Lalanne L. Disruption of information processing in schizophrenia: the time perspective. Schizophr. Res. Cogn. 2015;2:78–83. doi: 10.1016/j.scog.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giersch A., Lalanne L., Isope P. Implicit timing as the missing link between neurobiological and self disorders in schizophrenia? Front. Hum. Neurosci. 2016;10(303) doi: 10.3389/fnhum.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.M., Swets J.A. Signal detection theory and psychophysics. Society. 1966 [Google Scholar]
- Habeck C., Rakitin B., Steffener J., Stern Y. Contrasting visual working memory for verbal and non-verbal material with multivariate analysis of fMRI. Brain Res. 2012;1467:27–41. doi: 10.1016/j.brainres.2012.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B., Pratt J., Colzato L., Godijn R. Symbolic control of visual attention. Psychol. Sci. 2001;12:360–365. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Kapur S., Shitij Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A., Lindenmayer J.P. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br. J. Psychiatry. 1989 [PubMed] [Google Scholar]
- Lalanne L., Van Assche M., Giersch A. When predictive mechanisms go wrong: disordered visual synchrony thresholds in schizophrenia. Schizophr. Bull. 2012;38:506–513. doi: 10.1093/schbul/sbq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe E.K., Liu R.-J., Aghajanian G.K. Schizophrenia, Hypocretin (Orexin), and the Thalamocortical activating system. Schizophr. Bull. 2006;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-H., Bhaker R.S., Mysore A., Parks R.W., Birkett P.B.L., Woodruff P.W.R. Time perception and its neuropsychological correlates in patients with schizophrenia and in healthy volunteers. Psychiatry Res. 2009;166:174–183. doi: 10.1016/j.psychres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Los S.A., Kruijne W., Meeter M. Outlines of a multiple trace theory of temporal preparation article type: received on: accepted on: citation: outlines of a multiple trace theory of temporal preparation corresponding author. Front. Psychol. Cogn. 2014;5 doi: 10.3389/fpsyg.2014.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce R.D., Robert D. Oxford University Press; 1986. Response Times: Their Role in Inferring Elementary Mental Organization. [Google Scholar]
- Martin B., Franck N., Cermolacce M., Falco A., Benair A., Etienne E., Weibel S., Coull J.T., Giersch A. Fragile temporal prediction in patients with schizophrenia is related to minimal self disorders. Sci. Rep. 2017;7(8278) doi: 10.1038/s41598-017-07987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi P., Näätänen R. Foreperiod and simple reaction time. Psychol. Bull. 1981;89:133–162. [Google Scholar]
- Olk B. Effects of spatial, temporal and spatiotemporal cueing are alike when attention is directed voluntarily. Exp. Brain Res. 2014;232:3623–3633. doi: 10.1007/s00221-014-4033-7. [DOI] [PubMed] [Google Scholar]
- Orfei M.D., Spoletini I., Banfi G., Caltagirone C., Spalletta G. Neuropsychological correlates of cognitive insight in schizophrenia. Psychiatry Res. 2010;178:51–56. doi: 10.1016/j.psychres.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Orfei M.D., Piras F., Banaj N., Di Lorenzo G., Siracusano A., Caltagirone C., Bandinelli P.L., Ducci G., Spalletta G. Unrealistic self-overconfidence in schizophrenia is associated with left presubiculum atrophy and impaired episodic memory. Cortex. 2017;86:132–139. doi: 10.1016/j.cortex.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Penney T.B., Meck W.H., Roberts S.A., Gibbon J., Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2006;58:109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Pessiglione M., Seymour B., Flandin G., Dolan R.J., Frith C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J., Nitsch A.M., Miltner W.H.R., Straube T. Impaired representation of time in schizophrenia is linked to positive symptoms and cognitive demand. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F., Coull J.T. Implicit, predictive timing draws upon the same scalar representation of time as explicit timing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., del Pozo N.O., Gómez J., Guillen-Grima F., Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia. 2014;58:14–22. doi: 10.1016/j.neuropsychologia.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Reitan R. 1992. Trail Making Test: Manual for Administration and Scoring. [Google Scholar]
- Ristic J., Kingstone A. A new form of human spatial attention: automated symbolic orienting. Vis. Cogn. 2012;20:244–264. [Google Scholar]
- Roa Romero Y., Keil J., Balz J., Gallinat J., Senkowski D. Reduced frontal theta oscillations indicate altered crossmodal prediction error processing in schizophrenia. J. Neurophysiol. 2016;116 doi: 10.1152/jn.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum J.M. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch. Int. Pharmacodyn. Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- Roy M., Grondin S., Roy M.-A. Time perception disorders are related to working memory impairment in schizophrenia. Psychiatry Res. 2012;200:159–166. doi: 10.1016/j.psychres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Spalletta G., Piras F., Piras F., Caltagirone C., Orfei M.D. The structural neuroanatomy of metacognitive insight in schizophrenia and its psychopathological and neuropsychological correlates. Hum. Brain Mapp. 2014;35 doi: 10.1002/hbm.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P., Mishara A.L., Voss M., Heinz A., Sterzer P., Mishara A.L., Voss M., Heinz A. Thought insertion as a self-disturbance: an integration of predictive coding and phenomenological approaches. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenes S., Oberfeld D. Meta-analysis of time perception and temporal processing in schizophrenia: differential effects on precision and accuracy. Clin. Psychol. Rev. 2017 doi: 10.1016/j.cpr.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Tizard J., Venables P.H. Reaction time responses by schizophrenics, mental defectives and normal adults. Am. J. Psychiatry. 1956;112:803–807. doi: 10.1176/ajp.112.10.803. [DOI] [PubMed] [Google Scholar]
- Todd J., Michie P., Jablensky A. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin. Neurophysiol. 2003;114:2061–2070. doi: 10.1016/s1388-2457(03)00246-3. [DOI] [PubMed] [Google Scholar]
- Todd J., Michie P.T., Schall U., Karayanidis F., Yabe H., Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol. Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Turgeon M., Giersch A., Delevoye-Turrell Y., Wing A.M. Impaired predictive timing with spared time interval production in individual with schizophrenia. Psychiatry Res. 2012;197:13–18. doi: 10.1016/j.psychres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Umbricht D., Krljesb S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Vogeley K., Kupke C. Disturbances of time consciousness from a phenomenological and a neuroscientific perspective. Schizophr. Bull. 2007 doi: 10.1093/schbul/sbl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N.S., Gogtay N. Treatment of early onset schizophrenia: recent trends, challenges and future considerations. Front. psychiatry. 2012;3(29) doi: 10.3389/fpsyt.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F., Jablensky A. Time discrimination deficits in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 2009;167:12–20. doi: 10.1016/j.psychres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Zahn T.P., Rosenthal D., Shakow D. Effects of irregular preparatory intervals on reaction time in schizophrenia. J. Abnorm. Soc. Psychol. 1963;67:44–52. doi: 10.1037/h0049269. [DOI] [PubMed] [Google Scholar]
- Zelanti P.S., Droit-Volet S. Cognitive abilities explaining age-related changes in time perception of short and long durations. J. Exp. Child Psychol. 2011;109:143–157. doi: 10.1016/j.jecp.2011.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 The Delayed Item Recognition task. Participants had to hold in memory 1, 2 or 3 unnameable shapes for a maintenance interval (4 or 7 s) and to indicate whether or not the probe matched a shape included in the sample array using a two-choice button press at the onset of the response screen. The first level consisted of two blocks of 8 trials each and a fixed 7 s maintenance interval, while for the second and the third level four blocks of 8 trials each, with a fixed 7 s maintenance interval, were included (tot = 80 trials). Stimuli were selected pseudo-randomly from a pool of 400 shapes such that the same shape couldn't be presented twice. Percentage of probe-equal-to-sample and probe-different-from-sample trials was balanced across the experimental blocks, and the inter-stimulus interval was a fixed 3 s period of black screen.
Supplementary Fig.2 Temporal bisection, perceptual timing task -Results-. Individual mean proportion of longer than sample responses as a function of probe duration for HC and SZ.
Supplementary Fig.3 Temporal production, motor timing -Results-. Individual mean proportion of responses as a function of 200 ms time bins (range: 200-1600 ms) for HC and SZ.