ABSTRACT
Leptospira interrogans is the etiological agent of leptospirosis, a globally distributed zoonotic disease. Human infection usually occurs through skin exposure with water and soil contaminated with the urine of chronically infected animals. In this study, we aimed to quantitatively characterize the survival of Leptospira interrogans serovar Copenhageni in environmental matrices. We constructed laboratory microcosms to simulate natural conditions and determined the persistence of DNA markers in soil, mud, spring water and sewage using a quantitative PCR (qPCR) and a propidium monoazide (PMA)-qPCR assay. We found that L. interrogans does not survive at high concentrations in the tested matrices. No net growth was detected in any of the experimental conditions and in all cases the concentration of the DNA markers targeted decreased from the beginning of the experiment following an exponential decay with a decreasing decay rate over time. After 12 and 21 days of incubation the spiked concentration of 106 L. interrogans cells/ml or g decreased to approximately 100 cells/ml or g in soil and spring water microcosms, respectively. Furthermore, culturable L. interrogans persisted at concentrations under the limit of detection by PMA-qPCR or qPCR for at least 16 days in soil and 28 days in spring water. Altogether, our findings suggest that the environment is not a multiplication reservoir but a temporary carrier of L. interrogans Copenhageni, although the observed prolonged persistence at low concentrations may still enable the transmission of the disease.
IMPORTANCE Leptospirosis is a zoonotic disease caused by spirochetes of the genus Leptospira that primarily affects impoverished populations worldwide. Although leptospirosis is transmitted by contact with water and soil, little is known about the ability of the pathogen to survive in the environment. In this study, we quantitatively characterized the survival of L. interrogans in environmental microcosms and found that although it cannot multiply in water, soil or sewage, it survives for extended time periods (days to weeks depending on the matrix). The survival parameters obtained here may help to better understand the distribution of pathogenic Leptospira in the environment and improve the predictions of human infection risks in areas where such infections are endemic.
KEYWORDS: Leptospira, survival, soil, water, sewage, qPCR, persistence, soil microbiology, statistical modeling, waterborne pathogens
INTRODUCTION
Leptospirosis is a globally distributed life-threatening zoonotic disease that affects humans and other mammals. The current estimates put the number of cases over 1,000,000 annually with almost 60,000 deaths, making leptospirosis one of the most prevalent zoonotic diseases worldwide (1). Leptospirosis is caused by motile spirochetes from the genus Leptospira. Pathogenic leptospires colonize the kidneys of animal hosts and are chronically excreted with the urine. Humans and other animals are infected through abrasions or cuts in the skin or mucous membranes by contact with water or soil previously contaminated with infected urine (2). Leptospirosis outbreaks are reported seasonally in areas where the disease is endemic following rainfall events which lead to an increased human exposure to flood water, mud, and runoff (3–7). Therefore, the environment plays a central role in the spillover infections to humans and the circulation of the bacteria within the animal reservoir.
Currently, there is a very limited knowledge about the persistence of pathogenic leptospires in environmental matrices and the factors affecting their fate (8). Persistence ranging from a few hours to several months has been reported for different species and serovars in aquatic matrices such as tap, river, sea, and distilled water (9–13). Similarly, in soil the reported survival ranges span from a few hours to 193 days (14–18). A number of factors have been identified as affecting the persistence, including pH, salinity, soil moisture, temperature, and the presence of accompanying microorganisms (9, 10, 19–23). However, these studies were based on the isolation of leptospires by culture techniques or direct animal inoculation. These approaches are time-consuming, insensitive, and prone to errors such as the overgrowth by the autochthonous microbiota. Furthermore, their results were qualitative and left a knowledge gap regarding the quantitative survival dynamics of pathogenic leptospires in environmental matrices.
The ability of pathogenic leptospires to survive or even multiply in environmental matrices is particularly critical to determine the extent to which they serve as a reservoir of the disease. In this study, we aimed to quantify the survival of pathogenic leptospires in spring water, sewage, and soil under controlled laboratory conditions using quantitative PCR (qPCR). For this purpose, we selected two species: Leptospira interrogans serovar Copenhageni, a highly virulent serovar that has been associated with large seasonal outbreaks in urban slums in Brazil (5, 24), and Leptospira biflexa serovar Patoc, a saprophytic species. We constructed laboratory microcosms to simulate natural conditions and spiked them with known concentrations of leptospires. DNA was extracted from each microcosm over a period of 28 days and quantified by qPCR and/or propidium monoazide (PMA)-qPCR. Finally, we developed a statistical model to describe the fate of Leptospira DNA markers in the microcosms.
RESULTS
Decay model.
We developed a statistical model based on Weibull distributions to model the survival of Leptospira DNA markers in the microcosms. Starting with a full model, including the covariates species (L. interrogans and L. biflexa), medium (spring water, soil, mud, and sewage), treatment (sterile and nonsterile), and quantification method (qPCR and PMA-qPCR), the final model included species, medium, and quantification method (see Table S1 in the supplemental material). Treatment (sterile/nonsterile microcosm) did not contribute significantly to the model fit (P = 0.19) and was therefore not selected as a covariate in the final model. The modeled shape of the decay curves was lower than 1 (k = 0.715 ± 0.03), which indicated that the death hazard was not constant during the experimental time but instead decreased gradually after spiking. The modeled initial marker concentration (μ0) was 5.673 ± 0.041 log10 units, which reflected the loss of DNA due to the extraction procedure (see the supplemental material). Modeled decay parameters (ϕ and α) for Leptospira DNA markers in each of the experimental condition are presented in Table 1. All comparisons between markers below were based on this model.
TABLE 1.
Modeled decay parameters (ϕ and α) and 95% confidence intervals of L. interrogans and L. biflexa markers in spring water, soil, mud, and sewage microcosmsa
| Organism and method | Microcosm | ϕ values |
α values |
||||
|---|---|---|---|---|---|---|---|
| ϕ | LCI | UCI | α | LCI | UCI | ||
| L. interrogans | |||||||
| qPCR | Spring water | 51.5 | 38.4 | 68.9 | 0.90 | 0.80 | 0.95 |
| Brazilian soil | 16.3 | 13.2 | 20.3 | 0.08 | 0.03 | 0.17 | |
| Brazilian mud | 14.1 | 11.1 | 18.0 | 0.10 | 0.05 | 0.18 | |
| U.S. soil | 4.3 | 3.1 | 6.1 | 0.21 | 0.14 | 0.29 | |
| U.S. mud | 5.7 | 4.1 | 7.8 | 0.28 | 0.21 | 0.35 | |
| Sewage | 2.2 | 1.7 | 3.0 | 0.18 | 0.13 | 0.23 | |
| PMA-qPCR | Brazilian soil | 8.2 | 7.4 | 9.1 | 0.00* | 0.00 | 1.00 |
| Spring water | 25.8 | 22.5 | 29.7 | 0.00* | 0.00 | 1.00 | |
| L. biflexa | |||||||
| qPCR | Spring water | 42.2 | 27.4 | 64.8 | 0.96 | 0.92 | 0.98 |
| Brazilian soil | 13.4 | 9.2 | 19.5 | 0.21 | 0.11 | 0.37 | |
| Brazilian mud | 11.6 | 7.9 | 16.9 | 0.25 | 0.15 | 0.39 | |
| U.S. soil | 3.6 | 2.4 | 5.2 | 0.45 | 0.39 | 0.51 | |
| U.S. mud | 4.7 | 3.2 | 6.9 | 0.54 | 0.48 | 0.60 | |
| Sewage | 1.8 | 1.3 | 2.5 | 0.40 | 0.36 | 0.44 | |
Estimates with intervals that overlap are not significantly different at the 95% significance level. LCI, lower 95% confidence limit; UCI, upper 95% confidence limit. *, not significantly different from 0.
Differential persistence of Leptospira DNA markers in spring water and soil.
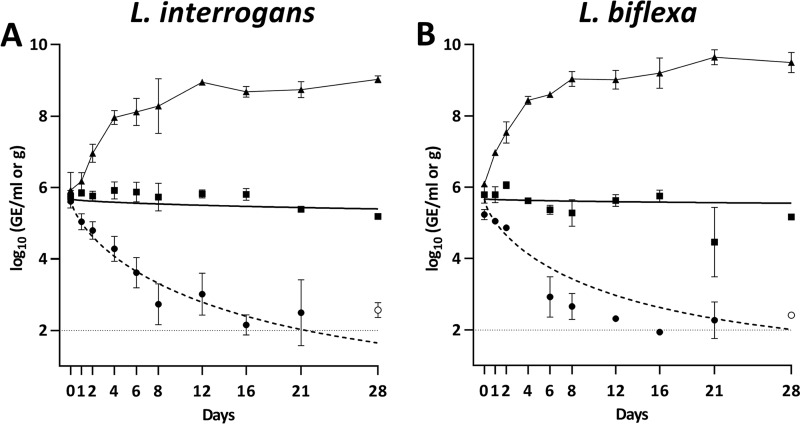
The concentration of markers for both L. interrogans and L. biflexa decreased in all the microcosms after spiking (Fig. 1). No differences were observed between decay rates of L. interrogans and L. biflexa markers in spring water or soil. In spring water, Leptospira markers presented an almost flat decay curve (ϕ = 51.5 and 42.2 for L. interrogans and L. biflexa, respectively) in which the DNA concentration had decreased by ∼0.5 log10 unit at the end of the experimental time. In contrast, the decay in soil microcosms was significantly faster (ϕ = 16.3 and 13.4, for L. interrogans and L. biflexa, respectively) with a rapid decrease during the first 8 days followed by stabilization at concentrations around 2.50 × 102 GE/g, marginally over the limit of detection. Leptospires cultured in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium did not show any time lag before entering the exponential phase, confirming that they were in good physiological conditions at the beginning of the experiments. Taken together, these results indicate that there was no net growth of Leptospira in spring water or soil.
FIG 1.
Persistence of L. interrogans (A) and L. biflexa (B) markers measured by qPCR in microcosms of spring water (squares), soil (circles), and EMJH media (triangles). The solid line represents the modeled decay curve in spring water and the dashed line in soil. Open symbols represent data points for which at least one observation was below the limit of detection. Error bars indicate standard deviations. The horizontal dashed line indicates limit of detection in soil samples.
Effect of moisture and soil characteristics on persistence.
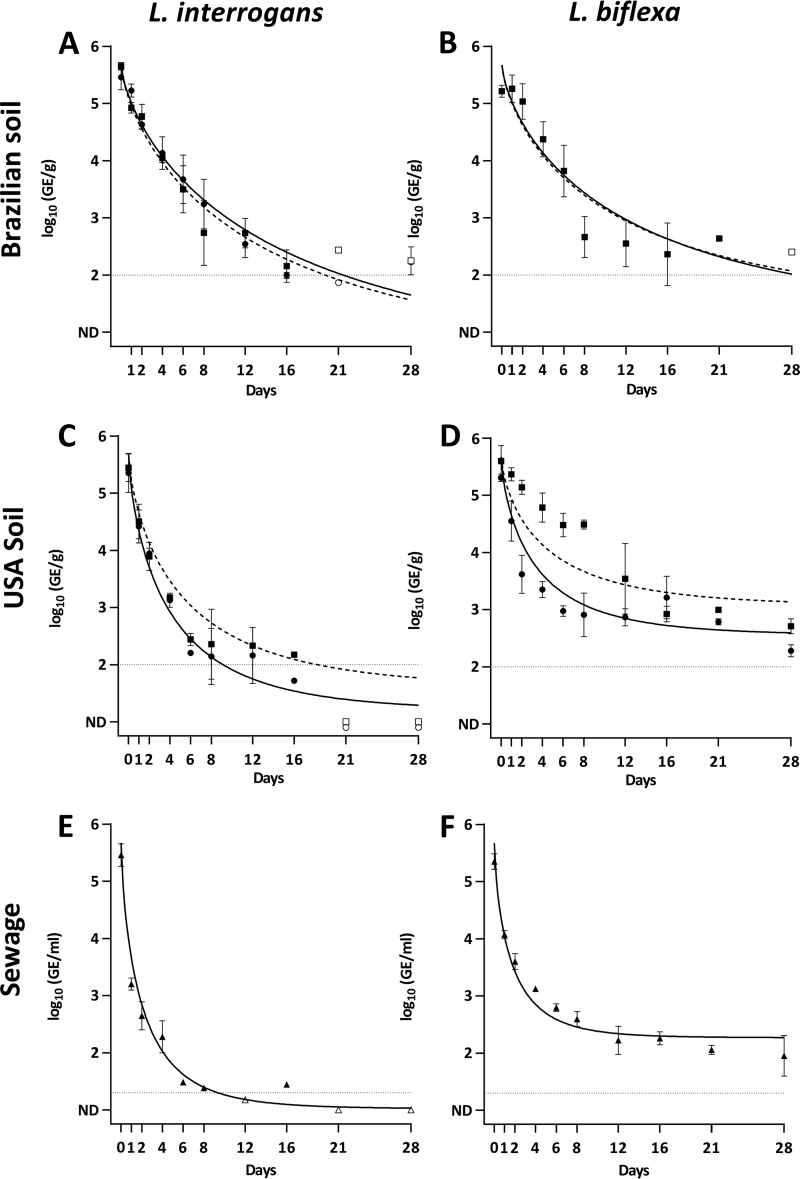
To evaluate the effect of soil moisture on the persistence of L. interrogans and L. biflexa markers, we compared their decay in two soils with different physicochemical characteristics adjusted to different moistures. We observed that the increase in moisture from field capacity to muddy conditions did not have any effect on the persistence of L. interrogans or L. biflexa in Brazilian and U.S. soils since the decay parameters ϕ and α were not statistically different (Fig. 2 and Table 1). The decay rates (ϕ) in Brazilian soil and mud were significantly smaller for L. interrogans and L. biflexa than the ones in U.S. soil. Conversely, the proportion of persistent markers (α) was significantly higher for both species in U.S. soil and mud than in Brazilian soil and mud, except for L. interrogans in Brazilian soil that showed no difference (Fig. 2 and Table 1). These observations indicated that moisture and intrinsic physicochemical characteristics of the soil such as pH, organic content, and texture affected the persistence of Leptospira.
FIG 2.
Persistence of L. interrogans and L. biflexa measured by qPCR in microcosms of Brazilian soil (A and B), U.S. soil (C and D), and sewage (E and F). In soil microcosms, circles denote soil adjusted to field capacity, and squares denote mud soils. Sewage samples are represented by triangles. The solid line represents the modeled decay curve in field capacity soil, and the dashed line represents the modeled decay curve in mud soils. Open symbols represent data points for which at least one observation was below the limit of detection. Error bars indicate standard deviations. The horizontal dashed line indicates the limit of detection.
Persistence of Leptospira DNA markers in sewage.
In sewage microcosms, Leptospira markers presented a rapid decay (ϕ = 2.23 and 1.83 for L. interrogans and L. biflexa, respectively), significantly faster than the decays observed in other media (Fig. 2E and F; Table 1). In addition, we observed that L. interrogans markers could only be consistently quantified above the limit of detection for 8 days (Fig. 2E) as opposed to L. biflexa, which was detected until the end of the experiment (Fig. 2F). This result is consistent with the estimated decay parameter (α) that indicated that a larger proportion of L. biflexa markers than L. interrogans persisted beyond the experimental time (Table 1). Thus, the experimental data suggest that L. biflexa survives better than L. interrogans in sewage.
Persistence of L. interrogans cells measured by PMA-qPCR in soil and spring water.
To determine whether the leptospiral DNA markers were suitable surrogates for live cells, we monitored the decay curves of heat-killed L. interrogans and L. biflexa in spring water and Brazil soil. In spring water, both L. interrogans and L. biflexa markers showed an almost flat decay curve indicating that the DNA from dead cells was being degraded at a very slow pace (see Fig. S1 in the supplemental material). The long persistence of DNA in spring water evidenced that the markers were not suitable surrogates for live cells. In contrast, in soil the persistence of DNA from heat-killed cells was shorter with a 3-log10 unit reduction in the first 4 to 6 days (see Fig. S1 in the supplemental material), which indicated that DNA from dead cells was being quickly degraded.
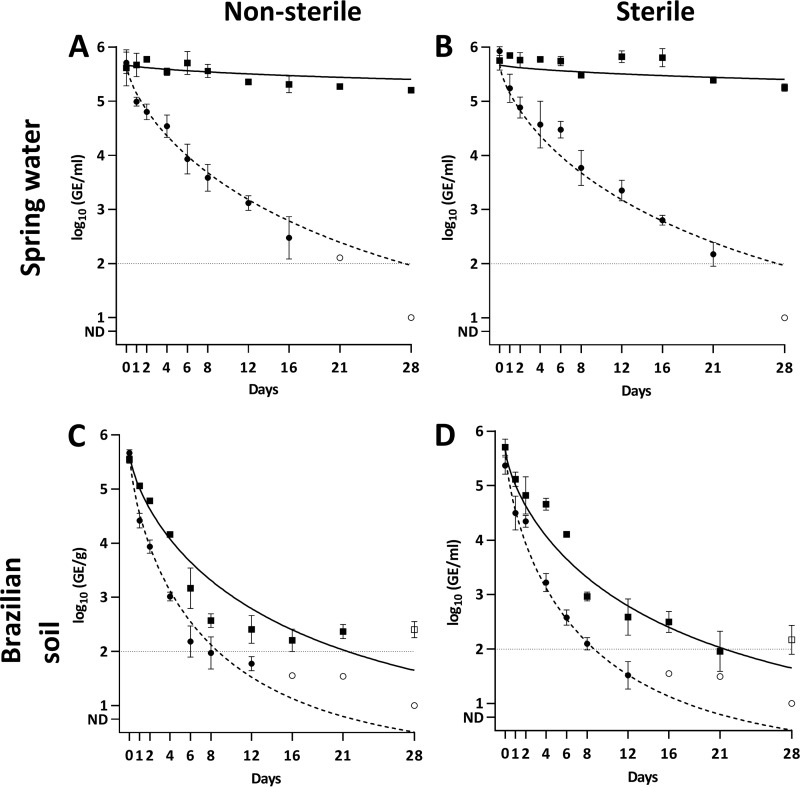
To discriminate between live and dead L. interrogans cells in the microcosms, we optimized a PMA-based qPCR (see the supplemental methods). Briefly, PMA-qPCR is a viability qPCR in which propidium monoazide (PMA), a DNA-binding dye, is added to the sample before DNA extraction. PMA penetrates cells whose membrane is compromised and binds covalently to DNA upon photoactivation interfering with its amplification. Therefore, the PMA treatment allows for the selective detection of DNA from membrane-intact “live” cells (25). After optimization of the PMA-qPCR procedure, we compared the persistence of markers in spring water and Brazilian soil using qPCR and PMA-qPCR. In addition, we tested sterile and nonsterile microcosms to explore the role of the autochthonous microbial communities on the survival. As anticipated by the previous experiment, the behavior of the markers in spring water was completely different when measured by qPCR or PMA-qPCR. In the first case, an almost flat decay was observed, indicating a long persistence of the markers in the system. Conversely, when using PMA-qPCR the decay rates of L. interrogans markers were higher (ϕ = 25.8), and there were no long-term persisting markers (α not statistically different from 0) (Fig. 3A and B; Table 1). These results indicate that L. interrogans cells were dying in the microcosm, but the extracellular DNA persisted for a long time in spring water without being degraded. Consequently, the qPCR measurement did not represent appropriately the fate of live L. interrogans cells in spring water. In addition, we did not observe major differences in decay parameters between sterile and nonsterile microcosms, which suggested that the spring water microbiota was not a major factor involved in the persistence of L. interrogans. Regarding the isolation of cells by culture, positive results were obtained in all sterile and nonsterile microcosms up to day 21. At day 28, only two replicates each showed still positive results, in agreement with the results obtained with PMA-qPCR (Table 2).
FIG 3.
Persistence of L. interrogans measured by qPCR and PMA-qPCR in sterile and nonsterile microcosms. (A and B) Spring water; (C and D) Brazilian soil. Squares denote measurements by qPCR, and circles denote measurements by PMA-qPCR. The solid line represents the modeled curve for qPCR measurements, and the dashed line represents the modeled curve for PMA-qPCR measurements. Open symbols represent data points for which at least one observation was below the limit of detection. Error bars indicate standard deviations. The horizontal dashed line indicates the limit of detection.
TABLE 2.
Proportion of positive cultures of L. interrogans Copenhageni from spring water and Brazilian soil microcosms obtained after 12 daysa
| Microcosm | Condition | No. of positive samples/total no. of samples tested at: |
||
|---|---|---|---|---|
| 16 days | 21 days | 28 days | ||
| Spring water | Sterile | 3/3 | 3/3 | 2/3 |
| Nonsterile | 3/3 | 3/3 | 2/3 | |
| Brazilian soil | Sterile | 2/3 | 1/3 | 0/3 |
| Nonsterile | 2/3 | 0/3 | 0/3 | |
All culture attempts before day 16 were successful.
In Brazilian soil, the decay of markers measured by PMA-qPCR was also faster than that measured by qPCR (ϕ = 8.2 and 16.3, respectively). At days 16 and 21 we detected markers by qPCR in all the experiments in both sterile and nonsterile microcosms, but when using PMA-qPCR, most replicates were negative (Fig. 3C and D), in agreement with the prediction of the model that no cells were long-term persistent (α = 0). Overall, these results showed that DNA markers persisted better than live L. interrogans cells in soil. However, as opposed to spring water, the decay shape was similar. Indeed, the average difference between the concentrations quantified by qPCR and PMA-qPCR before reaching the detection limit is 0.69 ± 0.34 log10 unit, with a maximum of 1.15 log10 units at day 4 (Fig. 3C). These relatively small differences indicated that qPCR could be used as a reasonable surrogate for live cells in soil, although it may overestimate the concentration of live cells. Furthermore, L. interrogans cells were consistently isolated by culture up to day 12 in all sterile microcosms and two of three nonsterile ones. At day 16, two sterile and two nonsterile microcosms were still positive (Table 2). Altogether, these data indicate that despite the decay of live L. interrogans in soil, culturable cells were still present in Brazilian soil after 16 days at concentrations under the limit of detection by PMA-qPCR.
DISCUSSION
In this study, we aimed to characterize the survival of the pathogenic spirochete L. interrogans Copenhageni in the environment. As with other environmentally dispersed bacteria, the transmission from host to host depends largely on the pathogen's ability to survive and remain infectious for a certain time outside the host. Our findings indicate that this species cannot survive at high concentrations in soil, spring water, or sewage. Yet, it exhibits a prolonged persistence in the environment that extends for over 3 weeks in soil and spring water.
L. interrogans did not show any net growth in the microcosms after spiking. The concentration of DNA markers decayed in all the environmental matrices. We observed that after approximately 14 and 5 days of incubation in spring water and soil microcosms, respectively, the initial concentration of 106 L. interrogans serovar Copenhageni cells/ml or g decreased by 3 log10 units (Table 1 and Fig. 3). This leads us to hypothesize that L. interrogans cannot multiply in the environment after excretion from its animal reservoirs, and thus the environment is not a reservoir from an epidemiological point of view but rather a temporary carrier of the pathogen. Consequently, although the environment is essential for the dispersion of the pathogen (4, 6, 26–29), it might not be sufficient to solely sustain the transmission cycle of the pathogen from animal to animal and the spillover infections to humans.
The experimental data collected in the microcosms fitted an exponential model with a decreasing decay rate over time. Various explanations have been proposed to explain this behavior that has been reported for Salmonella enterica, E. coli, Enterococcus spp., Campylobacter jejuni, and Bacteroidales, among other microorganisms (30–32), such as the regulation of the population though quorum-sensing (33). Alternatively, initial populations may be rapidly reduced due to predation or nutrient limitation until the carrying capacity of the ecosystem is reached (34). The mechanisms of survival of Leptospira in the environment are still poorly understood (8, 35), but the formation of biofilms and the interaction with other microorganisms (20, 23) could explain the decreasing decay rates observed. Unfortunately, after 12 and 21 days the concentrations in soil and spring water reached the limit of detection of the molecular methods (≈100 cells/g or ml) and the fate of L. interrogans serovar Copenhageni could not be monitored quantitatively thereafter. We succeeded, however, in culturing L. interrogans serovar Copenhageni from nonsterile field-capacity soils and spring water in all microcosms for at least 16 and 28 days, respectively (Table 2), even after the molecular approach yielded negative results. Furthermore, the decay model predicted that a small proportion of the initial population persisted in soil microcosms beyond the time at which the limit of detection was reached (Table 1; Fig. 3C). These low concentrations are consistent with those reported in waters and soils in surveys of the pathogen in areas of endemicity (27, 36, 37). Overall, this suggests that prolonged persistence at low concentrations may be sufficient to enable the transmission of the disease.
Our culture-based results for soil microcosms fall within the ranges reported previously for other L. interrogans serovars. For instance, L. interrogans serovar Australis survived for 15 days in moist silt loams from Australia (15), and L. interrogans serovar Hardjo was successfully cultured for up to 6 days from Malaysian moist loam and clay soils under natural shaded conditions (11). L. interrogans serovar Pomona survived for 42 days in saturated sterile soils under field conditions in New Zealand (16). Conversely, previous studies have found longer survival times in water than the ones reported in this study. L. interrogans serovar Icterohaemorragiae remained culturable for 316 days when incubated in spring water at 30°C (13). However, the addition of 1% of culture medium in their tested water clouds the interpretation of the results. In distilled water at lower temperature (20°C), L. interrogans serovar Canicola showed longer persistence (up to 110 days) (22). Despite the methodological differences with these studies, our results suggest that L. interrogans may have a shorter persistence in water at higher temperatures. This finding may be relevant to understand the role of freshwater and other aquatic matrices in the transmission dynamics of L. interrogans in tropical countries.
Sewage was not a suitable carrier for L. interrogans serovar Copenhageni. Although in this case our data were based exclusively on qPCR results, the decays of L. interrogans in sewage was faster than in soil and spring water (Fig. 2E). This decay is in agreement with Chang et al. (9), who reported that L. interrogans serovar Icterohaemorrhagiae was viable for no more than 2 to 3 days after spiking in undiluted sewage. Despite this relatively short persistence, exposure to sewage and flooding water after seasonal rainfall are widely recognized risk factors for leptospirosis infection (3, 38–40). Thus, the role that sewage plays in the pathogen mobilization, transportation, and distribution, especially during heavy rainfall and flooding events and, consequently, in the transmission of the disease, should not be disregarded.
Unexpectedly, L. biflexa serovar Patoc did not survive at high concentrations in any of the conditions tested (Fig. 2). Nevertheless, the decay of L. biflexa markers was slower than that of L. interrogans in soil and sewage (Fig. 2 and 3). Specifically, the proportion of markers that persisted beyond the experimental time (α) was significantly higher for L. biflexa than for L. interrogans (Table 1). This suggests that a small proportion of the inoculated L. biflexa persisted in soil and sewage 4 weeks postinoculation at low concentrations (<103 cells/g or ml). The concentration of naturally occurring L. biflexa or other saprophytic Leptospira in the environment has not been determined, but it is likely lower than the starting concentration of 107 cells/ml or g used in the microcosms to simulate the presumed excretion of L. interrogans by animal reservoirs. As indicated by the α parameters, L. biflexa may be decaying until the carrying capacity of the ecosystem is reached at concentrations close to the limit of detection by qPCR and surviving at low concentrations thereafter. Since we did not attempt to culture L. biflexa in the microcosms beyond the experimental time of 4 weeks, this hypothesis remains to be verified. Alternatively, L. biflexa may require a specific ecological niche to thrive different from the conditions tested here to simulate the environmental phase of L. interrogans in a tropical urban slum. Finally, future studies should preferably use recent isolates as the L. biflexa strain has been preserved in laboratory conditions for decades after isolation (41), which might have reduced its ability to thrive in the environment.
Microcosms are a convenient tool to study the persistence of microorganisms under controlled conditions, although the decay rates estimated using these systems might not perfectly predict the ones found in a variety of real settings (42, 43). For instance, we kept the microcosms at a constant incubation temperature of 29°C, which is a common temperature in standing water, small open sewers, and sun-exposed soil surfaces in tropical areas (44). In a real situation, however, this temperature may oscillate throughout the day and across different areas. Further studies should validate the results obtained here in more realistic settings that account for the variability of natural conditions. Another limitation of this study is that the long-term persistence of L. interrogans serovar Copenhageni seems to occur at concentrations close or below to the limit of detection by qPCR. Other alternative techniques should be developed to better explore the concentrations occurring in this phase of the decay and the mechanisms behind this survival. Moreover, future research should also explore the potential loss or reduction in infectivity of L. interrogans during its environmental phase using animal models of infection.
Despite these limitations, we succeeded in characterizing quantitatively the survival of L. interrogans in environmental matrices. Our results showed that L. interrogans exhibits a prolonged survival in the environment for periods ranging from a few days in sewage to at least 4 weeks in spring water. Although it does not survive at high concentrations in the environment, small subpopulations might persist in concentrations below 100 cells/g or ml for a prolonged time. Since the infectious dose in humans and animal reservoirs is unknown, the role that these small populations play in the spillover infections to humans and the maintenance of the pathogen within the animal reservoir should not be underestimated. Altogether, our results provide novel information that may have important ramifications regarding the life cycle of pathogenic Leptospira. The decay parameters reported here need to be integrated into models of the distribution of pathogenic Leptospira in the environment to improve the predictions of human infection risks and inform public health interventions to reduce the transmission of leptospirosis.
MATERIALS AND METHODS
Bacterial strains and culture.
Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 (45) and Leptospira biflexa serovar Patoc strain Patoc1 (41) were cultured in liquid EMJH medium (46, 47) in agitation (100 rpm) at 29°C for 3 to 5 days. A late-exponential culture was used in all the assays. After the incubation, 5 ml of the culture were centrifuged at 4,000 × g for 5 min, and the pellet was washed twice with the same volume of sterile spring water. The number of cells was determined using a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA) under dark-field microscopy, and the culture was adjusted to a concentration of 108 cells/ml with sterile spring water. For experiments requiring heat-killed cells, cultures were placed at 80°C for 15 min in a water bath and immediately cooled at room temperature for 20 min.
Soil and water samples.
The persistence of Leptospira spp. was investigated in two soils: a sandy loam soil (60% sand, 35% silt, and 5% clay, with 3.17% organic matter) collected in an urban slum in Salvador (Bahia, Brazil) and a loam soil (40% sand, 35% silt, and 25% clay, with 12.3% organic matter) collected in New Haven, CT. In addition, two water matrices were evaluated: bottled spring water obtained from a local retailer and sewage collected from the New Haven wastewater facility after the bar screen and grit removal. For the sterile controls, spring water was autoclaved once at 121°C for 20 min, and soil was autoclaved three times with 24 h of incubation at 29°C between cycles.
Microcosms.
Microcosms were prepared by distributing either 40 g of soil or 40 ml of water or sewage in sterile Pyrex glass beakers. The surface of the microcosm was spiked by dispersing droplets of Leptospira spp. suspensions to achieve a concentration of 106 cells/g or ml and thoroughly mixed. The volume of spiking suspension varied to adjust the moisture of the soils to 25 and 35% for the Brazilian and U.S. soils, respectively, which corresponded approximately to their field capacity. To create mud conditions, soil moisture was increased to 35 and 45%, respectively. After spiking, microcosms were thoroughly homogenized, sealed with plastic paraffin film to protect them from external inputs and prevent evaporation, and placed in a humid thermostatic chamber at 29°C under dark conditions. Samples of 1 g or 1 ml were withdrawn from each microcosm at 0, 1, 2, 4, 6, 7, 12, 16, 21, and 28 days for a total of 10 sampling time points. A growth control was carried out using EMJH medium instead of the environmental matrix. All microcosms were conducted in three independent biological replicates for L. interrogans serovar Copenhageni and in two for L. biflexa serovar Patoc.
DNA extraction methods.
Three DNA extraction methods for both spring water and soil samples were evaluated and compared (see the supplemental methods). Based on these results, soil samples and sewage were subsequently extracted using a Power Soil DNA isolation kit (Mo Bio), with minor modifications. Spring water and EMJH samples were extracted using a bead beating method with CTAB (cetyltrimethylammonium bromide) and phenol-chloroform/isoamyl alcohol. For the PMA assays, spring water was extracted with an automated Maxwell 16 cell DNA purification kit (Promega).
qPCR assays.
lipL32 gene was selected as a marker for L. interrogans and quantified using a TaqMan assay described elsewhere (48) with minor modifications (27). rpoB gene was selected as a marker for L. biflexa and was quantified using a newly designed SYBR green reaction (see the supplemental methods). Calibration curves were constructed using genomic DNA obtained from strains Fiocruz L1-130 or Patoc1 with concentrations ranging from 107 to 100 genomic equivalents (GE)/5 μl, based on its respective genome size (45, 49). A standard curve was run on each plate and used to transform quantification cycles (Cq) to concentrations (GE/reaction). Nontemplate controls were randomly included in all rows of each plate to discard the presence of contaminating DNA. All negative controls were negative in all cases. qPCR inhibition was monitored using a previously described internal amplification control plasmid tested in singleplex reactions (27). There was no evidence of inhibition of the molecular assays. See the supplemental material for further details on the qPCR assay, calibrators, and inhibition assay.
Isolation of Leptospira cells by culture.
From soil microcosms, 1 g of sample was mixed for 1 h with 4 ml of phosphate-buffered saline in a horizontal mixer, followed by sedimentation of the big particles for 30 min. Then, 3 ml of the supernatant was recovered and inoculated into 3 ml of 2× concentrated EMJH supplemented with 500 μl of a 10× concentrated antimicrobial combination (sulfamethoxazole, 400 μg/ml; trimethoprim, 200 μg/ml; amphotericin B, 50 μg/ml; fosfomycin, 4 mg/ml; 5-fluoroacil, 1 mg/ml) (50). From the spring water microcosm, a 1-ml sample was inoculated into 5 ml of EMJH liquid medium. When a culture showed contamination, s 1-ml sample was filtered through a 0.45-μm-pore size filter, and the filtrate was inoculated into 5 ml of EMJH containing the antibiotic cocktail. All cultures were incubated at 29°C with agitation and checked twice a week for Leptospira growth by dark-field microscopy. Samples were considered negative when no growth was observed after 30 days.
Detection of intact L. interrogans serovar Copenhageni cells.
The ability of PMA to selectively amplify DNA from membrane-intact L. interrogans cells in spring water and Brazilian soil was investigated. After optimization, a 60-min treatment with 5 μM PMA was selected for spring water and a 15-min treatment with 100 μM PMA was selected for Brazilian soil (see the supplemental methods).
Statistical modeling.
To model the survival curves of Leptospira markers and determine decay differences between species (L. interrogans and L. biflexa), medium (spring water, soil, mud and sewage), treatment (sterile and nonsterile), and quantification method (qPCR and PMA-qPCR), we assumed that cell death and marker disappearance from the microcosms were probabilistic events (51, 52). Thus, to describe the survival curves, a probabilistic Weibull distribution function was applied to the experimental data:
| (1) |
where k = 1 is a special case of the exponential function with a scale parameter ϕ. k defines the shape of the survival curve, and ϕ defines how stretched the shape is. Now, considering a set of experiments i = 1, ..., r, which is defined by the values of a set of covariates xi and the concentration of the bacteria were measured at each time tj (j = 1, 2, ..., m). Therefore, the concentration expected in a given time jth is based on the initial concentration (μ0), the proportion of cells that survive beyond the time of the experiments (α), and the family of survival functions, in this case Weibull distributions:
| (2) |
The effects of the covariates on the two parameters, ϕ and α, were explored to determine whether there were any differences between species, treatment, method of quantification, and substrates. Maximum-likelihood methods were used to estimate the parameters, assuming normality of the residuals. The log-likelihood function was optimized using the optim function in the R software package (53). See the supplemental methods for a full description of the survival model and the incorporation of samples below detection limits in the analysis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (R01 TW009504, U01 AI0088752, and R01 AI052473). G.G.P. received a Science Without Borders scholarship from the Brazilian Ministry of Education (CAPES-BEX 13715/13-5). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00507-18.
REFERENCES
- 1.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. 2015. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis 9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko AI, Goarant C, Picardeau M. 2009. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desvars A, Jégo S, Chiroleu F, Bourhy P, Cardinale E, Michault A. 2011. Seasonality of human leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS One 6:e20377. doi: 10.1371/journal.pone.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger D, Baroux N, Grangeon J-P, Ko AI, Goarant C. 2014. El Niño southern oscillation and leptospirosis outbreaks in New Caledonia. PLoS Negl Trop Dis 8:e2798. doi: 10.1371/journal.pntd.0002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD, Riley LW. 1999. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet 354:820–825. [DOI] [PubMed] [Google Scholar]
- 6.Lau CL, Watson CH, Lowry JH, David MC, Craig SB, Wynwood SJ, Kama M, Nilles EJ. 2016. Human leptospirosis infection in Fiji: an eco-epidemiological approach to identifying risk factors and environmental drivers for transmission. PLoS Negl Trop Dis 10:e0004405. doi: 10.1371/journal.pntd.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goarant C, Laumond-Barny S, Perez J, Vernel-Pauillac F, Chanteau S, Guigon A. 2009. Outbreak of leptospirosis in New Caledonia: diagnosis issues and burden of disease. Trop Med Int Health 14:926–929. doi: 10.1111/j.1365-3156.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 8.Barragan V, Olivas S, Keim P, Pearson T. 2017. Critical knowledge gaps in our understanding of environmental cycling and transmission of Leptospira spp. Appl Environ Microbiol 83:e01190-17. doi: 10.1128/AEM.01190-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SL, Buckingham M, Taylor MP. 1948. Studies on Leptospira iceterohaemorrhagiae. IV. Survival in water and sewage: destruction in water by halogen compounds, synthetic detergents, and heat. J Infect Dis 82:256–266. [DOI] [PubMed] [Google Scholar]
- 10.Smith CE, Turner LH. 1961. The effect of pH on the survival of leptospires in water. Bull World Health Organ 24:35–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Khairani-Bejo S, Bahaman AR, Zamri-Saad M, Mutalib AR. 2004. The survival of Leptospira interrogans in the Malayan environment. J Anim Vet Adv 3:123–129. [Google Scholar]
- 12.Saito M, Miyahara S, Villanueva SYAM, Aramaki N, Ikejiri M, Kobayashi Y, Guevarra JP, Masuzawa T, Gloriani NG, Yanagihara Y, Yoshida S. 2014. PCR and culture identification of pathogenic Leptospira from coastal soil in Leyte, Philippines after a storm surge during Super Typhoon Haiyan (Yolanda). Appl Environ Microbiol 80:6926–6932. doi: 10.1128/AEM.02568-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre-Fontaine G, Aviat F, Thorin C. 2015. Waterborne leptospirosis: survival and preservation of the virulence of pathogenic Leptospira spp. in fresh water. Curr Microbiol 71:136–142. doi: 10.1007/s00284-015-0836-4. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki W, Ringen LM. 1957. Some effects of various environmental conditions on the survival of Leptospira Pomona. Am J Vet Res 18:219–223. [PubMed] [Google Scholar]
- 15.Smith DJ, Self HR. 1955. Observations on the survival of Leptospira australis A in soil and water. J Hyg (Lond) 53:436–444. doi: 10.1017/S0022172400000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellstrom JS, Marshall RB. 1978. Survival of Leptospira interrogans serovar Pomona in an acidic soil under simulated New Zealand field conditions. Res Vet Sci 25:29–33. [PubMed] [Google Scholar]
- 17.Saito M, Villanueva SYAM, Chakraborty A, Miyahara S, Segawa T, Asoh T, Ozuru R, Gloriani NG, Yanagihara Y, Yoshida SI. 2013. Comparative analysis of Leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl Environ Microbiol 79:601–609. doi: 10.1128/AEM.02728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschner L, Maguire T. 1957. Survival of Leptospira outside their hosts. N Z Med J 56:385–391. [PubMed] [Google Scholar]
- 19.Parker J, Walker M. 2011. Survival of a pathogenic Leptospira serovar in response to combined in vitro pH and temperature stresses. Vet Microbiol 152:146–150. doi: 10.1016/j.vetmic.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Barragan VA, Mejia ME, Trávez A, Zapata S, Hartskeerl RA, Haake DA, Trueba GA. 2011. Interactions of Leptospira with environmental bacteria from surface water. Curr Microbiol 62:1802–1806. doi: 10.1007/s00284-011-9931-3. [DOI] [PubMed] [Google Scholar]
- 21.Henry RA, Johnson RC. 1978. Distribution of the genus Leptospira in soil and water. Appl Environ Microbiol 35:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trueba G, Zapata SS, Madrid K, Cullen P, Haake D. 2004. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int Microbiol 7:35–40. [PubMed] [Google Scholar]
- 23.Kumar KV, Lall C, Raj RV, Vedhagiri K, Vijayachari P. 2015. Coexistence and survival of pathogenic leptospires by formation of biofilm with Azospirillum. FEMS Microbiol Ecol 91:fiv051. doi: 10.1093/femsec/fiv051. [DOI] [PubMed] [Google Scholar]
- 24.Gouveia EL, Metcalfe J, de Carvalho ALF, Aires TSF, Caetano Villasboas-Bisneto J, Queirroz A, Santos AC, Salgado K, Reis MG, Ko AI. 2008. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis 14:505–508. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkuipou-Kenfack E, Engel H, Fakih S, Nocker A. 2013. Improving efficiency of viability-PCR for selective detection of live cells. J Microbiol Methods 93:20–24. doi: 10.1016/j.mimet.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Hagan JE, Moraga P, Costa F, Capian N, Ribeiro GS, Wunder EA, Felzemburgh RDMM, Reis RB, Nery N, Santana FS, Fraga D, dos Santos BL, Santos AC, Queiroz A, Tassinari W, Carvalho MS, Reis MG, Diggle PJ, Ko AI. 2015. Spatiotemporal determinants of urban leptospirosis transmission: four-year prospective cohort study of slum residents in Brazil. PLoS Negl Trop Dis 10:e0004275. doi: 10.1371/journal.pntd.0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanovas-Massana A, Costa F, Riediger IN, Cunha M, de Oliveira D, Mota DC, Sousa E, Querino VA, Nery N, Reis MG, Wunder EA, Diggle PJ, Ko AI. 2018. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. Water Res 130:176–184. doi: 10.1016/j.watres.2017.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agampodi SB, Dahanayaka NJ, Bandaranayaka AK, Perera M, Priyankara S, Weerawansa P, Matthias MA, Vinetz JM. 2014. Regional differences of leptospirosis in Sri Lanka: observations from a flood-associated outbreak in 2011. PLoS Negl Trop Dis 8:e2626. doi: 10.1371/journal.pntd.0002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amilasan AST, Ujiie M, Suzuki M, Salva E, Belo MCP, Koizumi N, Yoshimatsu K, Schmidt WP, Marte S, Dimaano EM, Villarama JB, Ariyoshi K. 2012. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg Infect Dis 18:91–94. doi: 10.3201/eid1801.101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers SW, Donnelly M, Peed L, Kelty CA, Mondal S, Zhong Z, Shanks OC. 2011. Decay of bacterial pathogens, fecal indicators, and real-time quantitative PCR genetic markers in manure-amended soils. Appl Environ Microbiol 77:4839–4848. doi: 10.1128/AEM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae S, Wuertz S. 2012. Survival of host-associated Bacteroidales cells and their relationship with Enterococcus spp., Campylobacter jejuni, Salmonella enterica serovar Typhimurium, and adenovirus in freshwater microcosms as measured by propidium monoazide-quantitative PCR. Appl Environ Microbiol 78:922–932. doi: 10.1128/AEM.05157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae S, Wuertz S. 2009. Rapid decay of host-specific fecal Bacteroidales cells in seawater as measured by quantitative PCR with propidium monoazide. Water Res 43:4850–4859. doi: 10.1016/j.watres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Ishino G, Matsuba T, Takayama H, Ishida S. 1999. Survival of bacteria at a subfreezing temperature (−1°C). Yonago Acta Med 42:147–152. [Google Scholar]
- 34.Easton JH, Gauthier JJ, Lalor MM, Pitt RE. 2005. Die-off of pathogenic Escherichia coli O157:H7 in sewage contaminated waters. J Am Water Resour Assoc 41:1187–1193. doi: 10.1111/j.1752-1688.2005.tb03793.x. [DOI] [Google Scholar]
- 35.Hu W-L, Pappas CJ, Zhang J-J, Yang Y-Y, Yan J, Picardeau M, Yang XF. 2017. The EbpA-RpoN regulatory pathway of the pathogen Leptospira interrogans is essential for survival in the environment. Appl Environ Microbiol 83:e02377-. doi: 10.1128/AEM.02377-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganoza CA, Matthias MA, Collins-Richards D, Brouwer K, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM. 2006. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med 3:1329–1340. doi: 10.1371/journal.pmed.0030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider AG, Casanovas-Massana A, Hacker KP, Wunder EA, Begon M, Reis MG, Childs JE, Costa F, Lindow JC, Ko AI. 2018. Quantification of pathogenic Leptospira in the soils of a Brazilian urban slum. PLoS Negl Trop Dis 12:e0006415. doi: 10.1371/journal.pntd.0006415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. 2008. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis 2:e228. doi: 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felzemburgh RDM, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AXTO, Fraga D, Santana FS, Mohr S, Dos Santos BL, Silva AQ, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. 2014. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the leptospira agent. PLoS Negl Trop Dis 8:e2927. doi: 10.1371/journal.pntd.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangkanakul W, Tharmaphornpil P, Plikaytis BD, Bragg S, Poonsuksombat D, Choomkasien P, Kingnate D, Ashford DA. 2000. Risk factors associated with leptospirosis in Northeastern Thailand, 1998. Am J Trop Med Hyg 63:204–208. doi: 10.4269/ajtmh.2000.63.204. [DOI] [PubMed] [Google Scholar]
- 41.Babudieri B. 1961. Studio serologico del gruppo Semaranga-Patoc di Leptospira biflexa, p 408–414. In XI Congress of the Italian Society for Microbiology, Cagliari-Sassari, Italy. [Google Scholar]
- 42.Green HC, Shanks OC, Sivaganesan M, Haugland RA, Field KG. 2011. Differential decay of human faecal Bacteroides in marine and freshwater. Environ Microbiol 13:3235–3249. doi: 10.1111/j.1462-2920.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- 43.Ballesté E, Blanch AR. 2010. Persistence of Bacteroides species populations in a river as measured by molecular and culture techniques. Appl Environ Microbiol 76:7608–7616. doi: 10.1128/AEM.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson GW. 1984. Field guide to soils and the environment applications of soil surveys. Springer, Dordrecht, Netherlands. [Google Scholar]
- 45.Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, Haake DA, Verjovski-Almeida S, Hartskeerl RA, Marques MV, Oliveira MC, Menck CF, Leite LC, Carrer H, Coutinho LL, Degrave WM, Dellagostin OA, El-Dorry H, Ferro ES, Ferro MIT, Furlan LR, Gamberini M, Giglioti EA, Goes-Neto A, Goldman GH, Goldman MHS, Harakava R, Jeronimo SMB, Junqueira-de-Azevedo ILM, Kimura ET, Kuramae EE, Lemos EGM, Lemos MVF, Marino CL, Nunes LR, de Oliveira RC, Pereira GG, Reis MS, Schriefer A, Siqueira WJ, Sommer P, Tsai SM, Simpson AJG, Ferro JA, Camargo LEA, Kitajima JP, Setubal JC, Van Sluys MA. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol 186:2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson RC, Harris VG. 1967. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J Bacteriol 94:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellinghausen HC, McCullough WG. 1965. Nutrition of Leptospira Pomona and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am J Vet Res 26:45–51. [PubMed] [Google Scholar]
- 48.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. 2009. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis 64:247–255. doi: 10.1016/j.diagmicrobio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, Creno S, Kuczek ES, Bommezzadri S, Davis JC, McGrath A, Johnson MJ, Boursaux-Eude C, Seemann T, Rouy Z, Coppel RL, Rood JI, Lajus A, Davies JK, Médigue C, Adler B. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3:e1607. doi: 10.1371/journal.pone.0001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakraborty A, Miyahara S, Villanueva SYAM, Saito M, Gloriani NG, Yoshida S-I. 2011. A novel combination of selective agents for isolation of Leptospira species. Microbiol Immunol 55:494–501. doi: 10.1111/j.1348-0421.2011.00347.x. [DOI] [PubMed] [Google Scholar]
- 51.Peleg M, Cole MB. 1998. Reinterpretation of microbial survival curves. Crit Rev Food Sci Nutr 38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- 52.van Boekel MAJ. 2002. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int J Food Microbiol 74:139–159. doi: 10.1016/S0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 53.R Core Team. 2013. R: a language and environment for statistical computing, 3.1. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.