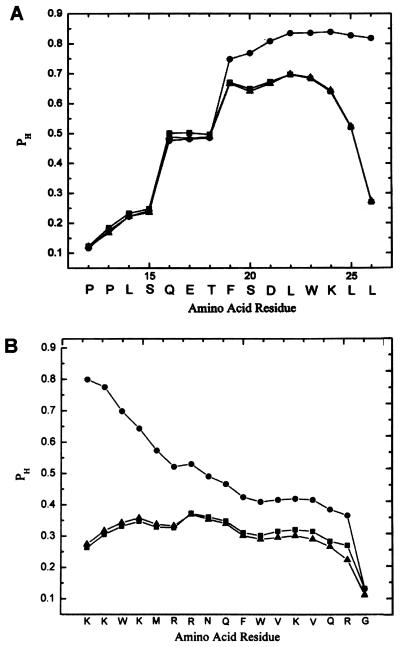
Figure 1.
(A) Helix probability (PH) profiles for the p53 12–26 peptide with no leader sequence (filled squares), with positively charged antennapedia leader sequence (filled triangles) on its amino-terminal end and with the leader sequence on its carboxyl terminal end (filled circles). (B) Helix probability profile for the antennapedia leader sequence alone (filled triangles), attached to the amino-terminal end of the p53-12–26 peptide (filled squares) and attached to the carboxyl terminal end (filled circles). Profiles were computed by using experimentally determined helix–coil parameters including the effects of charges as described by Vasquez et al. (9, 10).