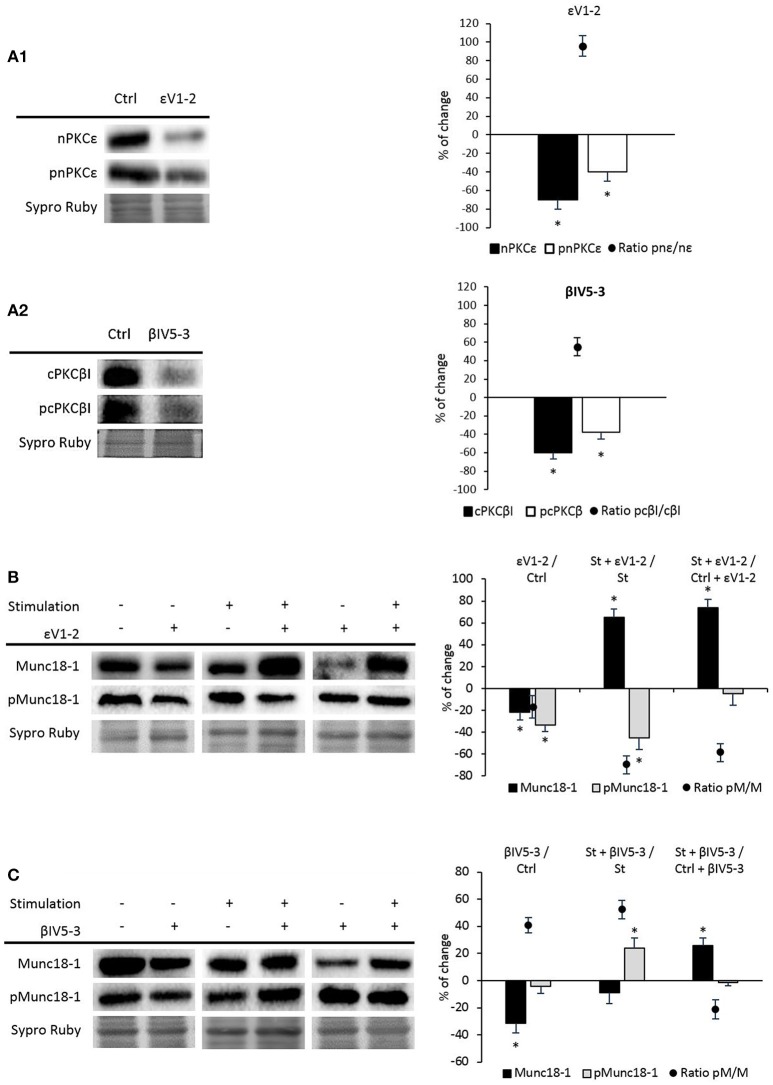
Figure 4.
nPKCε and cPKCβI regulate synaptic activity-induced Munc18-1 phosphorylation. (A1,A2) Representative Western blot bands and quantification show that εV1-2 and βIV5-3 peptides inhibit the presence of nPKCε and cPKCβI and their phosphorylation levels under basal conditions. (B) Representative Western blot bands and quantification show that under basal conditions both Munc18-1 and pMunc18-1 significantly decrease (εV1-2/Ctrl: muscles in basal conditions vs. basal conditions preincubated with εV1-2 peptide). Moreover, in both (St + εV1-2/ St: synaptic activity compared with the εV1-2 peptide) and (St + εV1-2/ Ctrl + εV1-2: basal conditions compared with stimulated samples; both treated with εV1-2 peptide) pMunc18-1/Munc18-1 ratios significantly decrease. (C) Representative Western blot bands and quantification show that in both (βIV5-3/Ctrl: muscles in basal conditions vs. preincubated with βIV5-3 peptide) and (St+βIV5-3/St: synaptic activity compared with the βIV5-3 peptide) conditions, the ratio of pMunc18-1/Munc18-1 significantly increases. Moreover, in (St+βIV5-3/Ctrl+βIV5-3: basal conditions compared with stimulated samples; both treated with βIV5-3 peptide) pMunc18-1/Munc18 ratio is significantly decreased. Data are mean percentage ± SEM, *p < 0.05 (n = 5).