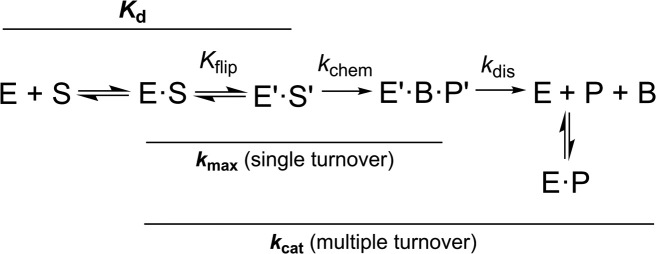
Figure 2.
Minimal kinetic mechanism for the TDG reaction. Association of TDG (E) and DNA substrate (S) gives a collision complex (E·S), and the reversible nucleotide flipping step (Kflip), involving conformational changes in E and S, gives the reactive enzyme-substrate complex (E′·S′). Cleavage of the N-glycosyl bond and addition of the (water) nucleophile in the chemical step (kchem) gives the ternary product complex (E′·B·P′). Dissociation of E′·B·P′ likely involves rapid release of the excised base (B) and much slower release (kdis) of abasic DNA (P). TDG is severely inhibited by abasic DNA but does not bind to the nucleobases it removes from DNA (51). Solid lines denote reaction steps that contribute to enzymatic rate constants (kmax, kcat), and the dissociation constant (Kd) for the reactive (E′·S′) complex. Note that catalytic turnover (kcat) is influenced by multiple steps, including dissociation of the initial product complex (kdis) and subsequent product inhibition.