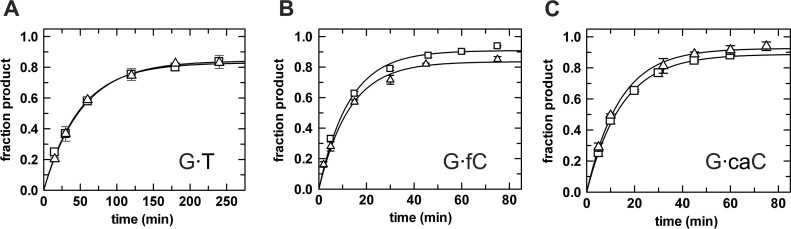
Figure 3.
Single turnover kinetics experiments (37°C) give the maximal glycosylase (base excision) activity of TDG∼SUMO-1 (squares) and TDG∼SUMO-2 (triangles). (A) Activity of sumoylated TDG (5 uM) and G·T substrate (1 uM); fitting to Equation (1) gives kmax = 0.020 ± 0.001 min−1 for TDG∼SUMO-1 and kmax = 0.019 ± 0.001 min −1 for TDG∼SUMO-2. (B) Activity of sumoylated TDG (1.5 uM) and G·fC substrate (0.5 uM); kmax = 0.079 ± 0.007 min−1 for TDG∼SUMO-1 and kmax= 0.078 ± 0.008 min−1 for TDG∼SUMO-2. (C) Sumoylated TDG (1.5 uM) acting on a G·caC substrate (0.5 uM); kmax= 0.069 ± 0.002 min−1 for TDG∼SUMO-1 and kmax = 0.074 ± 0.003 min−1 for TDG∼SUMO-2.