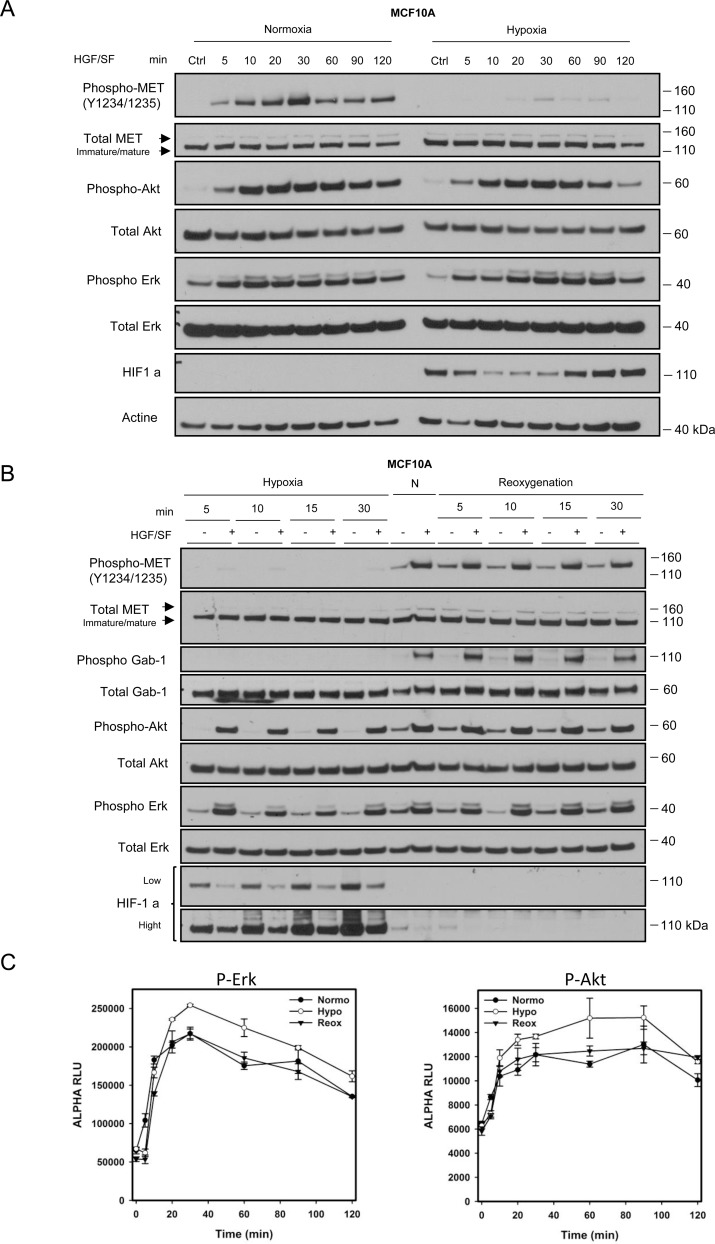
Figure 2. Dynamics of the hypoxia-triggered decrease in MET phosphorylation and its reversal upon reoxygenation.
(A) MCF10A cells were incubated under normoxia or hypoxia for 1 h. They were then treated, under the same oxygen pressure, with 10 ng/mL HGF/SF for 5, 10, 20, 30, 60, 90 and 120 minutes. A control (Ctrl) without any HGF/SF stimulation was also performed. The same amount of protein was analyzed by western blotting with antibodies directed against: phosphorylated residues in the MET kinase domain, the MET kinase domain, the hypoxia marker HIF1a, phosphorylated Akt, Akt, Erk2, phosphorylated Erk, and actine. The positions of prestained molecular weight markers are indicated. Arrows indicate the positions of precursor and mature full-length MET. (B) MCF10A cells were incubated under hypoxia for 5, 10, 15 or 30 minutes. Another set of cells were incubated under hypoxia for 1 h and then returned to normoxia for 5, 10, 15 or 30 min (re-oxygenation). A control under normoxic (N) conditions was also included. The same amount of protein was analyzed by western blotting as previously described with the addition of GAB1 and its phosphorylated form. (C) MCF10A cells were placed under normoxic or hypoxic conditions for 1 h or hypoxia for 1 h and then normoxia for 10 minutes (reoxygenation). Cells were then treated at the indicated time with 10 ng/mL of HGF/SF. Cell lysates were incubated for AlphaScreen specific phospho-Erk and phospho-Akt quantitation. Error bars represent standard deviations (n = 3; ± SD).