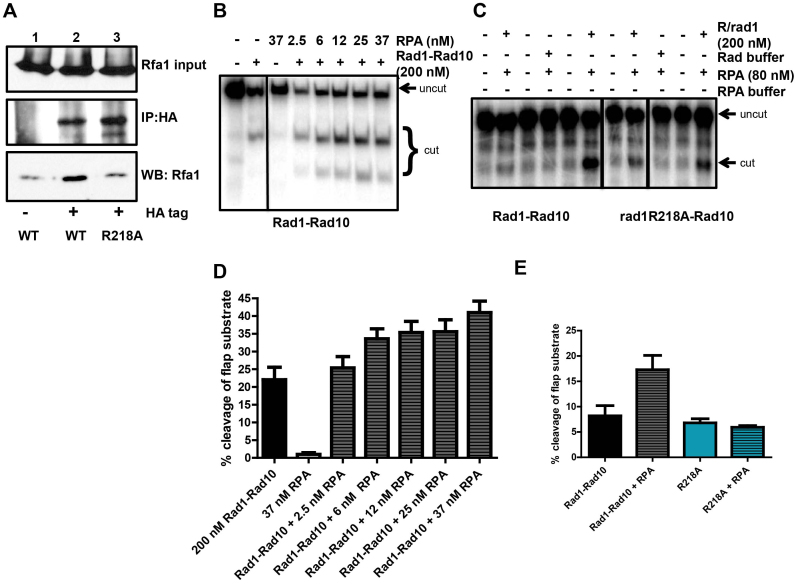
Figure 11.
rad1R218A disrupts interaction with RPA. (A) Co-immunoprecipitation experiments from cells. Rad1-HA or rad1R218A-HA was immunoprecipitated with α-HA antibody and associated proteins were probed by western blot, using α−Rfa1 antibody. (B) A Representative gel of the endonuclease activity of purified His–Rad1–Rad10 (200 nM) on 3′ flap substrates in the absence or presence of increasing concentrations of RPA. (C) Representative gel of His–rad1R218A–Rad10 endonuclease activity in the absence or presence of RPA. (D) Quantification of multiple RPA titration experiments, using multiple different preparations of His–Rad1–Rad10 and RPA. (E) Quantification of stimulation of cleavage activity by RPA. Note that while the absolute activity of Rad1–Rad10 and rad1R218A–Rad10 in the absence and presence of RPA varies, the relative levels remain consistent. For both panels B and C, the reactions shown were all run on the same gel; some unrelated lanes were cropped out of the images.