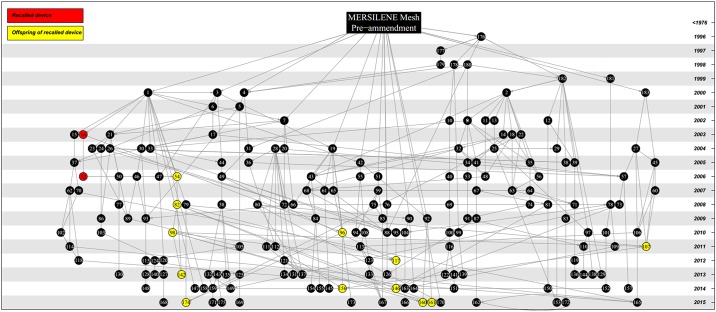
Fig 3. The ancestral device network of Mersilene Surgical Mesh manufactured by Ethicon Inc.
Mersilene Mesh has led to 183 descendent devices. Devices in the ancestral network that have since been recalled for ‘design and material related flaws’ (n = 2) are highlighted in red. Devices that are descended from recalled devices by substantial equivalency chains (n = 12) are highlighted in yellow (see S2 Data for devices).