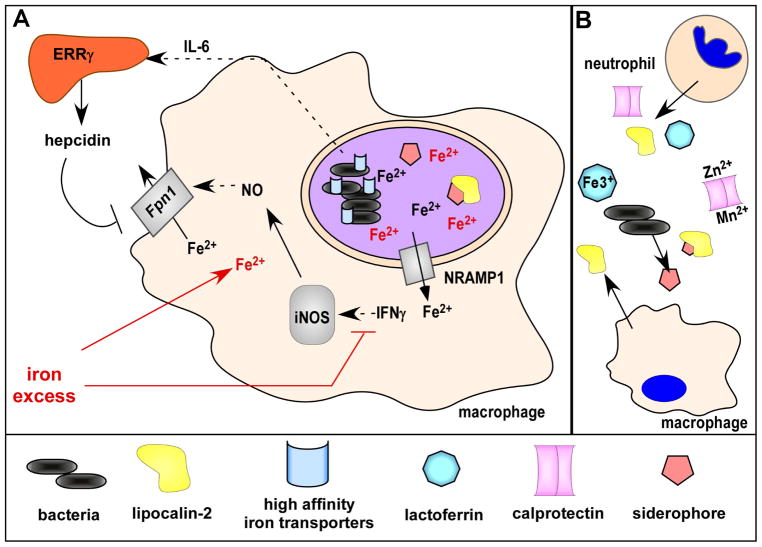
Figure 2. Nutritional immunity.
(A) During infection by intracellular pathogens, macrophages express NRAMP1 to export divalent metals, including iron, from bacteria-containing endosomes. Lipocalin-2 expression is upregulated to bind to bacterial siderophores and Fpn1 is produced to export cytosolic iron, overall limiting pathogen access to iron. Iron homeostasis is linked to other arms of the macrophage defense, as Fpn1 expression is further enhanced by interferon gamma (IFNγ) signaling to increase nitric oxide (NO) production via inducible nitric oxide synthase (iNOS). To compete for iron, intracellular bacteria express high affinity metal transporters and siderophores. Extraintestinal invasion by the bacterium S. Typhimurium additionally induces macrophage IL-6 production, resulting in hepcidin release from the liver through ERRγ signaling. Hepcidin increases Fpn1 degradation and limits iron export. Iron excess (shown in red), either through iron overload or over-supplementation, may subvert host defenses by interfering with IFN signaling and by increasing bacterial access to iron. (B) To block access to metals to extracellular bacteria, neutrophils release lipocalin-2, lactoferrin, and calprotectin while macrophages release lipocalin-2. Calprotectin binds and sequesters multiple metals including zinc and manganese. Lactoferrin binds primarily iron.