Abstract
Inflammatory markers have been shown to predict neurocognitive outcomes in aging adults; however, the degree to which peripheral markers mirror the central nervous system remains unknown. We investigated the association between plasma and CSF markers of inflammation, and explored whether these markers independently predict CSF indicators of Alzheimer’s disease (AD) pathology or neuronal damage. Plasma and CSF samples were analyzed for inflammatory markers in a cohort of asymptomatic older adults (n=173). CSF samples were analyzed for markers of AD pathology (Aβ42, phosphorylated tau [p-tau], sAPP-β) or neuronal damage (total tau; neurofilament light chain [NFL])(n=147). Separate linear models for each analyte were conducted with CSF and plasma levels entered simultaneously as predictors and markers of AD pathology or neuronal damage as outcome measures. Strong associations were noted between CSF and plasma MIP-1β levels, and modest associations were observed for remaining analytes. With respect to AD pathology, higher levels of plasma and CSF IL-8, CSF MIP-1β, and CSF IP-10 were associated with higher levels of p-tau. Higher levels of CSF IL-8 were associated with higher levels of CSF Aβ1-42. Higher CSF sAPP-beta levels were associated with higher plasma markers only (IL-8; MCP-1). In terms of neuronal injury, higher levels of plasma and CSF IL-8, CSF IP-10, and CSF MIP-1β were associated with higher levels of CSF total tau. Exploratory analyses indicated that CSF Aβ42 modifies the relationship between plasma inflammatory levels and CSF tau levels. Results suggest that both plasma and CSF inflammatory markers independently relay integral information about AD pathology and neuronal damage.
Keywords: neuroinflammation, systemic inflammation, aging, preclinical AD, biomarkers, alzheimer’s
1. Introduction
Dysregulation of inflammatory cascades is considered to be a core pathological component of Alzheimer’s Disease (AD)[1]. Whereas basal levels of inflammation and pathogen-specific activation of the immune system are clearly advantageous to the survival of our species, sustained inflammation has cytotoxic effects that may result in neuronal cell damage and acceleration of neurodegenerative processes[2–5]. Prior studies have linked CSF[6–8] and blood[9–11] levels of inflammation to clinical outcomes in Mild Cognitive Impairment (MCI), and epidemiological studies suggest that elevations in inflammatory markers may even be evident decades prior to manifestation of clinical symptoms[12]. These studies collectively highlight that dysregulated inflammatory processes may play a pivotal role early in AD pathogenesis. Despite these observations, however, disentangling the specific role and sequential impact of immune dysfunction on AD pathogenesis has proven to be challenging, and may stem in part from limitations in our understanding of the neurobiological significance of current immune biomarkers[13, 14].
Specifically, it is unclear how well the most frequently used method of assessing inflammation, i.e. peripheral (blood) inflammation, reflects or maps on to the central nervous system (CNS) immune milieu. Although several studies have measured CSF and blood markers of inflammation in tandem[6, 7, 15], few have reported the strength of associations between the specimen types. One of the isolated studies to describe these associations in adults with AD showed a strong, positive correlation between CSF and plasma markers of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), and alpha(1)-antichymotrypsin (ACT)[16]; however, no study to our knowledge has systematically examined the association(s) between CSF and blood inflammation in healthy aging adults or preclinical AD. Moreover, the relative contribution of CNS versus peripheral inflammation to biomarker indicators of neuronal cell damage or AD pathology remains an unresolved question. A recent study by our colleagues demonstrated an association between higher levels of a CSF marker of inflammation (YKL-40) and higher levels of CSF neurofilament light chain and total tau in an aging cohort at risk for AD[17]; while this suggests that CNS inflammation may reflect evidence of neuronal damage, it does not pinpoint whether CNS inflammation is a more robust indicator of AD biomarkers and neuronal cell damage than peripheral inflammation. Development of novel in-vivo immune biomarkers for AD may depend upon a better understanding of the relationship between immune dysfunction and AD pathogenesis, which could establish earlier detection methods and new targets for future therapeutics.
The goal of this study was to address a gap in the immune-AD field by examining two primary objectives: 1) to evaluate the relationship between CSF and plasma markers of inflammation in an asymptomatic, aging cohort at risk for AD; and 2) to determine the extent to which CSF and plasma inflammatory levels independently predict biomarkers of AD pathology and neuronal cell damage. Based on prior AD literature suggesting potential alterations in blood-CSF permeability in aging[18, 19] as well as the corpus of neuroimmunology studies identifying direct and indirect mechanisms for communication between the CNS and periphery[20, 21], we anticipated that CSF and plasma levels would be correlated with each other; however, in an asymptomatic group, we hypothesized that the degree of association might represent only small to medium effects. In terms of plasma versus CSF inflammation and their relationships with markers of AD pathology and neuronal cell damage, we considered several competing hypotheses. First, if plasma inflammation reflects an indirect or possibly ‘diluted’ manifestation of CNS function, then controlling for CSF inflammation might result in a weak or nonexistent association between plasma inflammation and AD/neuronal biomarkers. In contrast, if plasma inflammation reflects distinct aspects of the CNS milieu, then we would expect associations with markers of AD pathology and neuronal cell damage to remain even when accounting for CSF inflammation. We elected to focus our analyses on inflammatory markers that were detectable in both CSF and plasma, thereby allowing for data-driven, head-to-head comparisons of the specimen types.
2. Methods
Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and were in accordance with U.S. federal regulations. All participants provided written informed consent.
2.1 Participants
Participants were asymptomatic middle-aged adults and older adults who were enrolled in the Wisconsin Alzheimer’s Disease Research Center (ADRC) clinical core. By design, the group was enriched for parental history of dementia presumed due to AD. This was ascertained by one or more of the following methods: self-report of parental history confirmed through the dementia questionnaire interview[22], available medical records of the parent’s clinical dementia work up and diagnosis, and/or neuropathology report indicating AD using standardized ADC criteria[23]. The Wisconsin ADRC cohorts include well-characterized participants who undergo cognitive testing and neurological and physical exams, and are subsequently reviewed by a multidisciplinary consensus panel. In addition, inclusion criteria consisted of normal cognitive function determined by comprehensive neuropsychological evaluation and consensus review; negative history of psychiatric or neurological disease or untreated depression; and no history of head trauma. Please see prior publications for further description of this cohort[17, 24]. Participants were also required to have previously undergone lumbar puncture and blood draws for CSF and plasma assays, respectively, and were excluded from the analysis if their CSF and plasma blood draws occurred greater than 6 months apart. The final sample size for the study was 173 participants, although sample sizes for the individual objectives of the study varied based on assays conducted and available lab values for CSF and plasma (see section 2.3 below; Table 1).
Table 1.
Demographic Characteristics and CSF Biomarker Profiles of Participants.
| N | Mean (SD) or % | |
|---|---|---|
| Characteristics | ||
|
| ||
| Age (Years) | 173 | 63.9 (7.1) |
| Gender (% Male) | 173 | 35% |
| Education (Years) | 173 | 16.3 (2.5) |
| MMSE (Total) | 173 | 29.2 (1.1) |
| APOE Status (% >= 1 E4 allele) | 173 | 36.40% |
| Family History of AD (% yes) | 173 | 70.50% |
|
| ||
| CSF AD Markers (pg/mL) | ||
|
| ||
| Aβ42 | 147 | 738.4 (201.5) |
| sAPP-β | 147 | 554.1 (202.6) |
| Phosphorylated Tau [P-tau] | 147 | 47.43 (18.1) |
|
| ||
| CSF Neuronal Integrity Markers (pg/mL) | ||
|
| ||
| Total Tau | 147 | 340.3 (168.5) |
| Neurofilament Light Chain [NFL] | 147 | 795.6 (636.0) |
|
| ||
| CSF Inflammatory Markers (pg/mL) | ||
|
| ||
| IL-6 | 167 | 1.1 (.5) |
| IL-8 | 173 | 42.4 (11.0) |
| IP-10 | 164 | 363.3 (210.0) |
| MCP-1 | 173 | 392.4 (102.7) |
| MIP-1β | 171 | 9.8 (3.3) |
|
| ||
| Plasma Inflammatory Markers (pg/mL) | ||
|
| ||
| IL-6 | 161 | 0.6 (.3) |
| IL-8 | 169 | 5.9 (2.0) |
| IP-10 | 163 | 393.7 (160.3) |
| MCP-1 | 171 | 222.2 (53.8) |
| MIP-1β | 168 | 55.3 (18.0) |
2.2. Genotyping
APOEε4 genotype was performed on a non-fasting blood sample collected at baseline, using standard PCR and DNA sequencing techniques. DNA extracted from whole blood was genotyped with use of a homogeneous Fluorescent Resonance Energy Transfer technology coupled to competitive allele specific PCR (LGC Genomics; Beverly, MA). Genotyping also was performed by the National Cell Repository for Alzheimer’s Disease (NCRAD). There was 100% concordance for APOE genotype between these analyses. Participants were categorized using a binary variable as an APOEε4 carrier or non-carrier.
2.3. Plasma and CSF Collection and Analysis
Plasma Collection
After collection, each blood sample was centrifuged at 2000× g for 15 minutes at 4°C with the resultant plasma divided into 500 μL aliquots and stored at −80°C.
CSF Collection
CSF was collected with a Sprotte 25-or 24-gauge spinal needle at the L3/4 or L4/5 using gentle extraction into polypropylene syringes. Samples were collected in the morning after a 12h fast. Approximately 22mL of CSF were inverted to avoid gradient, gently mixed and centrifuged at 2000g for 10 minutes. Supernatants were frozen in 0.5mL aliquots in polypropylene tubes and stored at −80°C.
Plasma and CSF Markers of Inflammation
All plasma and CSF assays were conducted following the manufacturer’s protocol for Human Chemokine Panel 1 V-PLEX Plus and Human Pro-Inflammatory Panel kits (Meso Scale Diagnostics, Rockville, MD). Each multiplex array was scanned using a MESO QuickPlex SQ 120. Manufacturer supplied software (Discover Workbench 4.0) was used to quantify the concentrations based on sample dilution and relative to the supplied in-assay standard curve. Nominal recovery for control levels remained between 111%–120%. Standard curve coefficient of variation (CVs) for patient sample detection range remained <15% with standard sample recovery at 100% (+/− 5%) across all plates.
CSF Biomarkers of AD Pathology and Neuronal Cell Damage
In terms of biomarkers of AD pathology (phosphorylated tau, P-Tau181 [p-tau]; Aβ42 and sAPPβ) and general markers of neuronal cell damage (total tau [T-Tau]; neurofilament light chain [NFL]), a subset of samples (n=147) were analyzed for p-tau, t-tau, Aβ42, and sAPPβ using commercially available enzyme-linked immunosorbent assay (ELISA) methods (INNOTEST assays, Fujiurebio, Ghent Belgium) as described previously in detail[25]. CSF NFL was measured with a sandwich ELISA method (NF-light ELISA kit, UmanDiagnostics AB, Umeå, Sweden). Board-certified laboratory technicians who were blinded to clinical diagnosis performed all analyses on one occasion. All samples were analyzed according to protocols approved by the Swedish Board of Accreditation and Conformity Assessment (SWEDAC) using one batch of reagents (intra-assay coefficients of variation <10%). Three individuals had p-tau or t-tau levels below the detectible threshold of our assays, and those values were assigned the lowest detectible value for each marker (15.6 ng/L for p-tau; 75 ng/L for t-tau).
Given that our primary study goal was to compare both CSF and plasma markers, we restricted the analysis to inflammatory markers wherein at least 70% data was available for both CSF and plasma, defined by detectability (within detection limits of assay) and CV’s (< or = 20% coefficient of variation). Although 20% is higher than classically defined 10% CV’s, we opted to be more inclusive in these analyses given that this is the first head-to-head comparison of a large number of CSF vs plasma inflammatory markers in asymptomatic aging adults. Note that results did not substantively change when we retrospectively restricted the analysis to individuals with 10% CV’s, although it did reduce the sample size. Using this data driven approach, five inflammatory markers met our criteria: MCP-1, MIP-1 beta, IP-10, IL-6 and IL-8 (see Supplementary Table 1a and Supplementary Table 1b for quantification details).
2.4 Statistical Analysis
Next, we faced a statistical modeling challenge as several of the analytes displayed isolated outliers (MIP-1 beta [plasma: 4 outliers; CSF: 2 outliers], IP-10 [plasma: 8 outliers; CSF: 9 outliers], IL-6 [plasma: 5 outliers; CSF: 6 outliers]; and IL-8 [plasma: 3 outliers]). To reduce bias in our approach, we elected to remove outlier data points that were outside of 3 x the interquartile range (i.e. levels greater than Quartile 3 + [3*Interquartile Range] or Quartile 1 – [3*Interquartile Range]). All results, including participant characteristics and CSF biomarker profiles (Table 1), exclude these outliers.
To examine the relationship between CSF and plasma levels of inflammation (Objective 1), simple bivariate correlation analyses were first conducted. To determine whether CSF and plasma markers of inflammation independently predict CSF markers of AD pathology or neuronal cell damage (Objective 2), we next set up a series of separate general linear models. All models were adjusted for age and sex. CSF markers of AD pathology and neuronal cell damage were analyzed at two separate times (i.e. single time point data collection, two separate batches), thus we also included CSF batch as a covariate. We checked for collinearity between CSF and plasma markers of inflammation using standard techniques (e.g. variance inflation factor [VIF]), and VIF was less than 2 for all models. A natural log transformation was applied to NFL values to correct for a right skew. Separate linear models for the five inflammatory markers (i.e. MCP-1, MIP-1 beta, IP-10, IL-6 and IL-8) were conducted with CSF and plasma levels for an individual marker entered simultaneously as predictors (e.g. MCP-1 plasma and MCP-1 CSF) and CSF markers of AD pathology as separate outcome variables. This was repeated for indices of neuronal damage. We subsequently included APOE gene status to determine if the presence or absence of at least 1 E4 allele impacted the results. All analyses were conducted using SAS 9.4.
3. Results
3.1. Association Between Demographics, CSF and Plasma Inflammatory Markers
As noted in Table 1, the participant sample was comprised of middle aged to older adults (mean age=63.9; SD=7.1) who were highly educated (mean education=16.3 years, SD=2.5) and predominantly female. The majority of individuals had a positive family history of Alzheimer’s disease (~70%), and APOE ε4 status was overrepresented compared to the typical aging population (~36%).
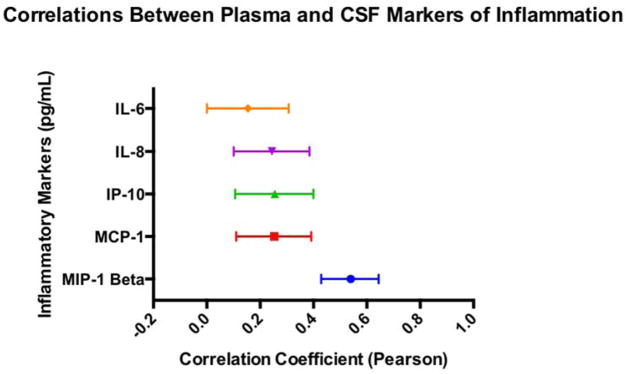
As shown in Figure 1, the relationship between CSF and plasma levels of inflammation varied by individual marker; stronger associations were noted between MIP-1β CSF and plasma levels (r=.55, p<.001), and modest associations between plasma and CSF levels were observed for IL-8 (r=.25, p=.001), IL-6 (r=.16, p=.05), MCP-1 (r=.28, p=.001) and IP-10 (r=.26, p=.001).
Figure 1.
Displays pearson correlations between plasma and CSF for each inflammatory marker. Bands represent a 95% confidence interval based on Fisher’s transformation.
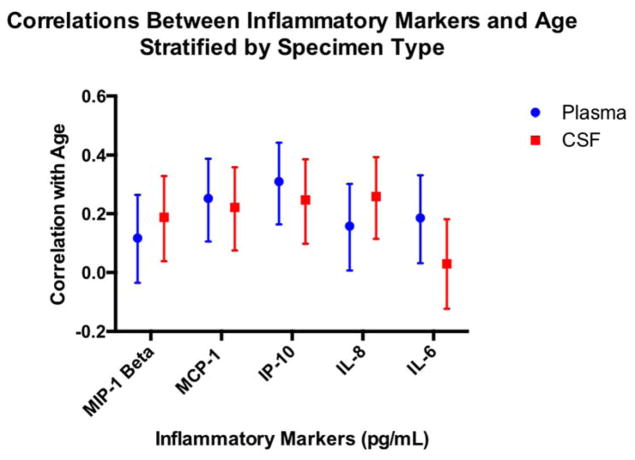
Age was associated with all inflammatory markers, with the exception of CSF IL-6 and plasma MIP-1β (p’s >.05; see Figure 2). Neither education level nor family history of AD diagnosis was significantly associated with inflammatory markers, irrespective of specimen type (all p’s greater than .05).
Figure 2.
Displays the pearson correlations between inflammatory markers and age as a function of specimen type (plasma; CSF); bars represent 95% confidence intervals based on Fisher’s transformation.
3.2. Relationship of Inflammation and CSF Markers of AD Pathology1: Head to Head Comparison of Plasma vs CSF Inflammation (Table 2)
Table 2.
CSF/Plasma Inflammation and CSF Markers of AD Pathology
| CSF p-Tau | CSF-Aβ42 | CSF sAPPβ | ||||
|---|---|---|---|---|---|---|
| Unstandardized Beta (Standard Error) | Unstandardized Beta (Standard Error) | Unstandardized Beta (Standard Error) | ||||
| 95% Confidence Interval; t-value | 95% Confidence Interval; t-value | 95% Confidence Interval; t-value | ||||
|
|
||||||
| Plasma | CSF | Plasma | CSF | Plasma | CSF | |
|
|
||||||
| IL-6 | 9.268 (4.989) | −1.993 (3.163) | 38.301 (59.207) | −22.299 (37.530) | 38.010 (59.063) | −29.342 (37.439) |
| 95% CI: −0.607 to 19.143; t=1.86 | 95% CI: −8.253 to 4.267; t=−0.63 | 95% CI: −78.886 to 155.489; t=0.65 | 95% CI= −96.581 to 51.984; t=−0.59 | 95% CI: −78.893 to 154.913; t=0.64 | 95% CI: −103.445 to 44.760; t=−0.78 | |
| IL-8 | 2.169 (0.702)** | 0.401 (0.125)** | 6.137 (8.771) | 3.810 (1.560)** | 23.466 (8.800)** | 0.755 (1.566) |
| 95% CI: 0.782 to 3.556; t=3.09 | 95% CI: 0.155 to 0.648; t=3.22 | 95% CI: −11.205 to 23.480; t=0.70 | 95% CI: 0.726 to 6.894; t=2.44 | 95% CI: 6.066 to 40.867; t=2.67 | 95% CI: −2.34 to 3.850; t=0.48 | |
| IP-10 | 0.001 (.009) | 0.022 (0.007)** | 0.068 (0.113) | 0.075 (0.086) | 0.139 (0.117) | 0.089 (0.089) |
| 95% CI: −0.017 to 0.020; t=0.15 | 95% CI: 0.008 to 0.0359; t=3.13 | 95% CI: −0.156 to 0.292; t=0.60 | 95% CI: −0.095 to 0.246; t=0.87 | 95% CI: −0.092 to .369; t=1.19 | 95% CI: −0.067 to 0.264; t=1.00 | |
| MCP-1 | 0.052 (0.029) | −0.004 (0.014) | 0.189 (0.347) | −0.023 (0.169) | 0.669 (0.340)* | 0.268 (0.165) |
| 95% CI: −0.006 to 0.109; t=1.77 | 95% CI: −0.032 to 0.024; t=−0.31 | 95% CI: −0.498 to 0.876; t=0.54 | 95% CI: −0.357 to 0.3116; t=−0.13 | 95% CI: −0.002 to 1.341; t=1.97 | 95% CI: −0.059 to 0.594; t=1.62 | |
| MIP-1β | −0.058 (0.095) | 1.541 (0.514)** | −0.334 (1.145) | 4.978 (6.215) | −0.512 (1.152) | 8.255 (6.253) |
| 95% CI: −0.245 to 0.129; t=−0.61 | 95% CI: 0.524 to 2.558; t=3.00 | 95% CI: −2.598 to 1.930; t=−0.29 | 95% CI: −7.312 to 17.268; t=0.80 | 95% CI: −2.790 to 1.767; t=−0.44 | 95% CI: −4.110 to 20.620; t=1.32 | |
|
|
||||||
p < .05;
p< .01
With respect to CSF markers of AD pathology, higher levels of IL-8 plasma and CSF (IL-8 plasma: beta=2.17, 95% confidence interval [CI]=0.78 to 3.56; p=.002; IL-8 CSF: beta= 0.40, 95%CI= 0.15 to 0.65; p=.002), MIP-1β CSF (beta=1.54, 95%CI= 0.52 to 2.56; p=.003), and IP-10 CSF (beta=0.02, 95%CI= 0.01 to 0.04; p=.002) were associated with higher CSF p-tau levels in separate models for each marker. IL-8 CSF (beta=3.81; 95% CI= 0.73 to 6.89; p=.02) was the sole inflammatory markers associated with CSF Aβ42, such that higher levels of IL-8 CSF were unexpectedly associated with higher levels of CSF Aβ42. Finally, higher levels of IL-8 plasma (beta=23.47; 95%CI=6.07 to 40.87; p=.009) and MCP-1 plasma (beta=0.67; 95%CI=0.00 to 1.34; p=.05) were associated with higher levels of CSF sAPP-β, but no associations were observed between CSF cytokines or chemokines and CSF sAPP-β.
3.3. Relationship of Inflammation with CSF Markers of Neuronal Injury: Head to Head Comparison of Plasma vs CSF Inflammation (Table 3)
Table 3.
CSF/Plasma Inflammation and CSF Markers of Neuronal Integrity
| CSF Total Tau | CSF Neurofilament (Log Transformed) | |||
|---|---|---|---|---|
| Unstandardized Beta (Standard Error) | Unstandardized Beta (Standard Error) | |||
| 95% Confidence Interval; t-value | 95% Confidence Interval; t-value | |||
|
|
||||
| Plasma | CSF | Plasma | CSF | |
|
|
||||
| IL-6 | 103.588 (46.376)* | −30.233 (29.396) | 0.2515 (0.103)* | −0.0414 (0.066) |
| 95% CI: 11.799 to 195.379; t=2.23 | 95% CI: −88.417 to 27.951; t=−1.03 | 95% CI: 0.0468 to 0.4561; t=2.43 | 95% CI: −0.1711 to 0.0883; t=−0.63 | |
| IL-8 | 13.966 (6.701)* | 3.526 (1.192)** | 0.0190 (0.016) | 0.0084 (0.003)** |
| 95% CI: 0.716 to 27.216; t=2.08 | 95% CI: 1.170 to 5.883; t=2.96 | 95% CI: −0.0119 to 0.0498; t=1.22 | 95% CI: 0.0029 to 0.0139; t=3.02 | |
| IP-10 | 0.154 (0.083) | 0.177 (0.063)** | 0.0006 (0.000)** | 0.0004 (0.000)** |
| 95% CI: −0.011 to 0.319; t=1.85 | 95% CI: 0.052 to 0.303; t=2.80 | 95% CI: 0.0002 to 0.0010; t=3.10 | 95% CI: 0.0001 to 0.0007; t=2.68 | |
| MCP-1 | 0.263 (0.273) | −0.048 (0.133) | 0.0006 (0.001) | 0.0002 (0.000) |
| 95% CI: −0.277 to 0.804; t=0.96 | 95% CI: −0.313 to .214; t=−0.36 | 95% CI: −0.0007 to 0.0018; t=0.91 | 95% CI: −0.0004 to 0.0008; t=0.70 | |
| MIP-1β | −0.444 (0.876) | 15.553 (4.753)** | 0.0026 (0.002) | 0.0048 (0.011) |
| 95% CI: −2.178 to 1.288; t=−0.51 | 95% CI: 6.154 to 24.953; t=3.27 | 95% CI: −0.0015 to 0.0067; t=1.26 | 95% CI: −0.0175 to 0.0271; t=0.42 | |
|
|
||||
p< .05;
p< .01
In terms of markers of neuronal injury, higher IL-8 CSF and plasma levels (IL-8 plasma: beta= 13.97; 95%CI= 0.72 to 27.22; p=.04; CSF: beta= 3.53, 95%CI= 1.17 to 5.88; p=.004), IP-10 CSF levels (beta=0.18, 95%CI=0.05 to 0.30; p=.006), IL-6 plasma levels (beta=103.59; 95%CI=11.80 to 195.38; p=.03), and MIP-1β CSF levels (beta=15.55; 95%CI= 6.15 to 24.95; p=.001) were associated with higher levels of total-tau. Higher levels of IP-10 plasma and CSF (IP-10 plasma, beta=0.0006; 95%CI= 0.0002 to 0.0009; p=.002; IP-10 CSF, beta=0.0004, 95%CI=0.0001 to 0.0007, p=.008), IL-6 plasma (beta=0.251, 95% CI=0.05 to 0.46, p=.02), and IL-8 CSF (beta=0.01, 95%CI=0.00 to 0.01, p=0.003) were independently associated with higher CSF NFL levels.
All results remained substantively unchanged when restricting the sample to clinical dementia rating (CDR) scale of 0 (removing 9 participants), with the exception of the relationship between IL-6 plasma and total tau; although the association remained in the same direction, the effect no longer reached statistical significance (p=.052).
3.4 Exploratory Analyses: The Impact of CSF Aβ42 levels on the Relationship Between Inflammatory Markers and Tau Levels
Standard biomarker analyses of AD pathology focus primarily on CSF levels of Aβ42, p-tau, and total tau, and have been included in research criteria for preclinical AD[27] and clinical diagnostic criteria for MCI and dementia due to AD[28, 29]. Based on both the utility of these markers in clinical diagnosis and current study results showing relatively larger effects of inflammatory markers on tau (p-tau; total tau) than Aβ42, we investigated in exploratory analyses whether lower CSF Aβ42 levels might alter the strength of the relationship between inflammatory markers and tau levels. This exploratory analysis thus addresses whether the relationship between higher inflammatory markers and higher tau markers of neuronal degeneration is more pronounced in the presence of increased amyloid deposition. In a series of linear models, we included an interaction term between CSF Aβ42 levels and individual inflammatory markers (plasma and CSF were evaluated in separate models, unlike analyses in section 3.3), with either CSF p-tau or CSF total tau as the primary outcome variables.
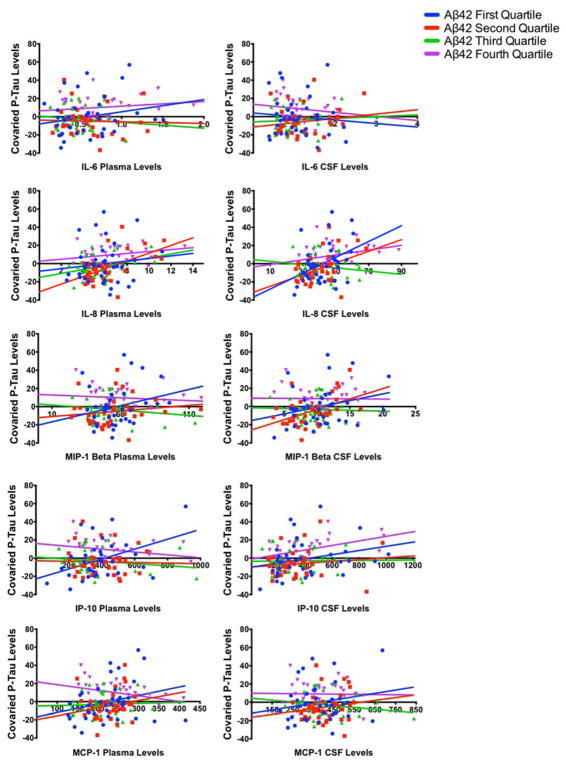
With respect to p-tau, significant interactions between CSF Aβ42 and plasma IP-10 (beta=−0.0001, SE=0.00004; t=−2.96; p=.004) as well as CSF Aβ42 and plasma MCP-1 (beta=−0.0004; SE=0.0001; t=−3.15; p=.002) predicting CSF p-tau levels were noted. A trend for an interaction between CSF Aβ42 and CSF IL-8 (beta= −0.001; SE=0.0006; t=−1.90; p=.06), predicting p-tau levels was also observed. To visualize and further elucidate the interactions, we divided CSF Aβ42 into quartiles and plotted the relationship between each inflammatory marker and p-tau levels as a function of CSF Aβ42 quartiles (see Figure 3). Results were in the expected direction, such that stronger positive correlations between inflammatory markers and CSF p-tau were observed when CSF Aβ42 levels were lower.
Figure 3.
Figure displays the association between inflammatory markers and p-tau levels as a function of CSF Aβ42 quartiles. P-tau levels were adjusted for demographics and APOE status. Significant interactions were observed for both plasma MCP-1 and plasma IP-10, such that stronger positive correlations between inflammatory markers and CSF p-tau were observed when CSF Aβ42 levels
Analyses were repeated with total tau levels, and while all results were in the same direction as p-tau levels, effects for IP-10 plasma were smaller. Significant interactions were only noted between CSF Aβ42 and plasma MCP-1 (beta=−0.004, SE=0.001; t=−3.13; p=.002) predicting higher CSF total-tau levels. Trends for an interaction between CSF Aβ42 and plasma IP-10 (beta=−0.0007, SE=0.0004; t=−1.82; p=.07) and CSF Aβ42 and plasma CSF IL-8 were also observed (beta=−.009, SE=0.006; t=−1.70, p=.07).
4. Discussion
In a cohort of asymptomatic older adults enriched for a family history of Alzheimer’s disease, we found modest associations between plasma and CSF levels of inflammation, although the relationship between plasma and CSF MIP-1β was quite strong. Importantly, results indicate that plasma and CSF markers of inflammation may relay independent and distinct information about AD pathology and neuronal damage in the absence of clinical symptoms. These findings collectively suggest that innate immune system dysregulation may be an early event in the AD pathogenic course, and that the role of inflammation may be particularly deleterious in the presence of Aβ42 deposition.
A systemic inflammatory response has been reported in both aging[30, 31] and AD[32–35], although the utility of plasma inflammation as an indicator of CNS immune dysregulation has been more controversial and remarkably understudied. One of the two primary goals of our study was to assess the association between CSF and plasma inflammation to clarify how well plasma inflammation reflects CNS inflammation. The correlations between CSF and plasma for several inflammatory analytes (MCP-1, IL-6, IL-8, and IP-10) suggest small to medium effects, whereas the relationship between CSF and plasma MIP-1β was notable for larger effects (r=.55). These results indicate that the level of correspondence between CSF and plasma may vary as a function of the specific analyte, although it’s unclear whether the strength of associations between CSF and plasma inflammation equate to more adverse clinical outcomes. Moreover, these results raise questions regarding the structural integrity of CNS-periphery boundaries, and whether specific inflammatory markers have varying degrees of permeability throughout the disease process.
In line with these questions, connections between the CNS and periphery in the context of aging and AD have stirred numerous debates, although it’s clear that CSF spaces (i.e. subarachnoid space and ventricles) do not display the same level of immune privilege as the parenchyma[21]. Moreover, the choroid plexus, which serves as a blood-CSF barrier (BCSFB) in addition to its role in CSF production, has been shown to conduct immunosurveillance and interface between the CNS and peripheral circulation[18]. Under physiological (i.e. non-pathological) conditions, the choroid plexus maintains tight junctions that prevent the passage of myeloid immune cells into the CNS; however, recent reports suggest that the BCSFB undergoes structural and functional alterations in the presence of[36] and possibly preceding[19] amyloid deposition. These alterations may have downstream effects on CSF-interstitial fluid exchange that could ultimately impact communication with the periphery[37]. Chemokines (i.e., a class of signaling proteins that are involved in chemotaxis) in particular may play a critical role in uni-directional or bi-directional communication between the CNS and periphery early on in the disease course. Prior studies have suggested that several markers from the CC and CXCL chemokine families, including IL-8 (CXCL8), MCP-1 (CCL2), and IP-10 (CXCL10), are upregulated and expressed in the CSF[38] and plasma[39] in the context of aging. Moreover, blood-borne chemokines have also been shown to induce alterations in the CNS in animal models, including declines in hippocampal neurogenesis and impairments in memory function[39, 40]. Interestingly, MIP-1β (CCL4; a chemoattractant for natural killer cells and instrumental in the innate immune response) and IL-8 (CXCL8) production in polymorphonuclear neutrophils has recently been shown to be very low in MCI and absent in AD dementia when exposed to lipopolysaccharide (LPS) stimulation[41]. Together, these studies may suggest dysregulation in innate immunity in response to early pathological changes, with subsequent decline in innate immunity as the disease progresses. Although evidence suggests that immune communication between the CNS and periphery is not only possible, but likely in pathological states, this does not resolve why isolated chemokines (MIP-1β) showed stronger associations between CSF and plasma than other inflammatory markers in our study, nor does it address the directionality of CNS-peripheral communication with respect to inflammation as a whole. Despite these unanswered questions, our results do point to some degree of overlap between CSF and plasma inflammation that should be explored further in future studies.
Directly relevant to the role of blood inflammation as an indicator of the CNS milieu is the extent to which CSF and plasma inflammatory levels independently predict biomarkers of AD pathology and neuronal cell damage. We initially hypothesized that if plasma inflammation was a downstream indicator of CNS alterations that it might not contribute unique variance to markers of neuronal integrity or AD pathology when compared to CSF. More specifically, given that CSF is thought to be a more proximal indicator of the CNS milieu, one might expect that CSF markers of inflammation would be stronger predictors of AD pathology and neuronal cell damage than plasma markers of inflammation. Results from our study suggest that both plasma and CSF markers of inflammation independently relay information about AD pathology and neuronal damage in head-to-head comparisons. With respect to indices of AD pathology (i.e. markers of amyloid deposition and phosphorylated tau), higher plasma and CSF levels of IL-8 were associated with higher levels of CSF p-tau, and higher levels of IL-8 were also associated with higher levels of CSF-Aβ42 (CSF only) and CSF-sAPPβ (plasma only). Interestingly, IL-8 was the only marker associated with Aβ42 levels, and was in a counterintuitive direction. Recent evidence from transgenic mice suggests that there may be a temporary increase in CSF Aβ42 levels prior to a precipitous decline[42], thus it is possible that this association reflects a very early alteration in the pathological cascade. Of note, higher levels of CSF IP-10 and CSF MIP-1β were also associated with higher CSF p-tau levels, again reiterating the potential role of chemokines in early stages of AD pathogenesis[43].
With respect to indices of general neuronal damage (total tau; NFL), we found independent associations between higher levels of CSF (IL-8, IP-10, and MIP-1β) and plasma (IL-6; IL-8) inflammation and higher levels of CSF total tau, as well as higher levels of plasma (IP-10; IL-6) and CSF (IP-10; IL-8) inflammation and higher levels of NFL. Overall, markers of inflammation displayed particularly consistent associations with levels of tau (p-tau and total tau) and NFL that were generally stronger than associations with Aβ42 levels, highlighting a potential role for both CSF and plasma inflammation as markers of underlying neuronal damage. Whether these associations suggest a specific relationship with neuronal damage in AD remains unclear. To clarify, whereas total tau and NFL reflect general markers of neuronal damage that have been associated with microglial alterations and inflammation in the context of a wide range of neurodegenerative diseases[44, 45], phosphorylated tau is a marker of neuronal damage that is specifically linked with AD pathology. Despite this distinction, they are all typically elevated in the CSF of AD and may be indicators of general disease progression or severity. Potentially consistent with our study findings, recent animal studies have uncovered a strong relationship between systemic inflammation and tau propagation. One of the earliest studies on this relationship found that secretion of the cytokine IL-1β from microglia was sufficient to increase tau phosphorylation in culture[46]. More recent studies have further demonstrated that dysregulated immune processes, including reactive microglia and altered chemokine receptors, precede and exacerbate phosphorylated tau aggregation in mouse models of AD[47, 48]. Thus, whereas the differential role of CSF versus plasma inflammation in predicting AD pathogenesis and progression remains unclear, the bourgeoning literature on tau propagation in tandem with our study results suggest that both central and systemic inflammation may contribute to the development of AD-specific tau pathology and impact neuronal integrity in aging adults.
Following up on our study results suggesting that CSF and plasma inflammation may reflect evidence of neuronal damage, we also explored whether the relationship between inflammatory markers and tau levels was impacted by the presence of Aβ42. We focused our analyses primarily on p-tau and total tau given their respective roles in current diagnostic guidelines for AD, although it is notable that associations with NFL were also quite strong. A significant interaction was observed between CSF Aβ42 levels and both plasma MCP-1 and plasma IP-10, such that the associations between higher levels of inflammation and higher levels of p-tau were stronger when CSF Aβ42 levels were lower. Similar patterns of associations were noted for total tau, although the effect sizes were notably smaller for the IP-10 x Aβ42 interaction. These findings suggest that the impact of pro-inflammatory cascades on neuronal integrity may be contingent upon the degree of amyloid deposition in the brain; however, it is important to highlight that in the context of a cross-sectional design and in a participant sample wherein the majority (~75%) of individuals did not meet CSF ptau/Aβ42 ratio cutoffs for preclinical AD, these findings may be interpreted in several ways, and thus warrant consideration of current theoretical frameworks. Recent literature suggests that inflammatory markers play an early pathogenic role in AD development[49], such that the presence of Aβ primes microglia and elicits the production of inflammatory mediators. In the context of chronic activation, microglia and astrocytes undergo morphological changes that can result in dysregulation of inflammatory feedback loops and reduction in critical trophic factors[1]. These dynamic alterations may aggravate tau propagation and ultimately lead to neuronal death. Thus, one interpretation of the literature and current data is that the temporal lag between Aβ deposition and clinical outcomes is in part driven by both a central and systemic dysregulation of the inflammatory response that occurs only after a critical threshold of amyloid deposition is reached. An additional consideration of the current data is that it may provide insight into the discordant literature on blood inflammation and clinical outcomes in typical aging studies[4, 50–52], as it may be that stronger associations between inflammation and aging variables are evident primarily in individuals with higher Aβ deposition.
In terms of study limitations, we utilized a data-driven approach to identify inflammatory markers of interest; although this permitted us to perform direct comparisons between CSF and plasma inflammatory markers, it also limited our ability to address potentially important markers that were detectable in one specimen type only. In addition, although CSF markers of AD pathology and neuronal integrity are considered a gold standard for diagnosis of preclinical and clinical AD, we also faced a potential bias in head-to-head comparisons, as CSF-CSF comparisons might be artificially stronger than plasma-CSF associations due to continuity in methods and individual test sensitivity. Cross-validation of test sensitivity for CSF and plasma inflammation methods may be fruitful in the future; nonetheless, the independent effects of both plasma and CSF inflammation in the context of these methods are encouraging. An additional consideration is that we used CSF levels as a proxy for the CNS milieu; however, there are still boundaries between the CSF and parenchyma (e.g. ependymal cell layers) that provide immunological privilege, thus it’s important to consider that CSF may not encapsulate the complexity of AD pathogenic processes in the CNS. As noted previously, although we have offered speculations as to potential mechanisms and points of interaction between the CNS and periphery, the cross-sectional design of the current study limits our ability to address directionality of communication between the nervous systems or causality with respect to the immune response. This study did not address recent history of infection or surgical procedures, which could impact both peripheral and central immune markers. Finally, based on our prior work demonstrating a lack of association between non-steroidal anti-inflammatory drugs [NSAIDs], circulating inflammatory markers, and clinical outcome measures in asymptomatic adults[53], we elected to not control for over the counter medications in our statistical models. Although we do not anticipate substantive differences in our cross-sectional findings based on NSAID use, it may be helpful for future longitudinal studies to address changes in NSAID use and dosing with respect to inflammation outcome measures. With respect to future studies, in order to disentangle the roles of CSF and plasma inflammation in the context of AD, longitudinal studies that include both preclinical and early AD (i.e. MCI due to AD) will be necessary. In addition, to determine whether the associations between inflammatory markers and CSF indicators of neuronal damage are specific to AD pathology, future studies should examine these markers in a) symptomatic AD and b) non-AD neurodegenerative phenotypes (e.g. frontotemporal dementia; Parkinson’s disease).
In summary, in a cohort of asymptomatic aging adults enriched for a family history of AD, modest associations were observed between plasma and CSF levels of inflammation, and results suggest that both plasma and CSF markers of inflammation independently relay integral information about AD pathology and neuronal damage. Results support a potential role for inflammatory cascades early in the AD pathogenic progress, prior to clinical manifestation of disease, and highlight that inflammation may be particularly deleterious in the presence of Aβ42 deposition. These findings add to a growing body of literature underscoring a complex relationship between systemic inflammation, central inflammation, and pathological outcomes.
Supplementary Material
Acknowledgments
This study was supported by NIH-NIA grants NIA K23AG042492 (PI: Bettcher), 1R01AG032289 (PI: Kramer), R01AG048234 (PI: Kramer), UCSF ADRC P50 AG023501, Wisconsin ADRC P50 AG033514 (PI: Asthana), R01AG037639 (PI: Bendlin), R01 AG021155 and R01 AG027161 (PI Johnson). HZ and KB are supported by grants from the Swedish Research Council. KB holds the Torsten Söderberg Professorship of Medicine and HZ is a Wallenberg Academy Fellow.
Footnotes
Based on CSF ptau/Aβ42 ratios, approximately 25% of the sample reached a clinically meaningful threshold based on cutoffs derived from the broader WADRC participant sample (clinical AD and low-risk controls).[26] Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, Bendlin BB, Asthana S, Johnson SC, Zetterberg H, Carlsson CM (Under Review) Age-accelerated cognitive decline in asymptomatic adults with CSF β-amyloid.
The authors have no other disclosures or conflicts of interest.
References
- 1.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 2.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 3.Eikelenboom P, Hoozemans JJ, Veerhuis R, van Exel E, Rozemuller AJ, van Gool WA. Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer’s disease. Alzheimers Res Ther. 2012;4:15. doi: 10.1186/alzrt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, Fenoglio C, Scarpini E. Inflammation in neurodegenerative disorders: friend or foe? Curr Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 6.Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiology of aging. 2010;31:1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PloS one. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonca VA, Gattaz WF, Forlenza OV. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2010;22:1305–1311. doi: 10.3233/JAD-2010-100921. [DOI] [PubMed] [Google Scholar]
- 11.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiology of aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 13.Bettcher BM, Kramer JH. Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: a mini-review. Clinical pharmacology and therapeutics. 2014;96:464–469. doi: 10.1038/clpt.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosano C, Marsland AL, Gianaros PJ. Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. Aging Dis. 2012;3:16–33. [PMC free article] [PubMed] [Google Scholar]
- 15.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Disease and Associated Disorders. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 17.Melah KE, Lu SY, Hoscheidt SM, Alexander AL, Adluru N, Destiche DJ, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Gleason CE, Dowling NM, Bratzke LC, Rowley HA, Sager MA, Asthana S, Johnson SC, Bendlin BB. Cerebrospinal Fluid Markers of Alzheimer’s Disease Pathology and Microglial Activation are Associated with Altered White Matter Microstructure in Asymptomatic Adults at Risk for Alzheimer’s Disease. J Alzheimers Dis. 2016;50:873–886. doi: 10.3233/JAD-150897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science (New York, NY) 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalbot S, Zetterberg H, Blennow K, Fladby T, Andreasen N, Grundke-Iqbal I, Iqbal K. Blood-cerebrospinal fluid barrier permeability in Alzheimer’s disease. J Alzheimers Dis. 2011;25:505–515. doi: 10.3233/JAD-2011-101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JM, Keefe RS, Mohs RC, Davis KL. A study of the reliability of the family history method in genetic studies of Alzheimer disease. Alzheimer Dis Assoc Disord. 1989;3:218–223. [PubMed] [Google Scholar]
- 23.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT National Institute on A, Alzheimer’s A. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, Hermann BP, Sager MA. Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dement Geriatr Cogn Disord. 2014;38:16–30. doi: 10.1159/000355682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hagerstrom D, Wollmer P, Minthon L, Hansson O. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 26.Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, Bendlin BB, Asthana S, Johnson SC, Zetterberg H, Carlsson CM. Age-accelerated cognitive decline in asymptomatic adults with CSF β-amyloid. doi: 10.1212/WNL.0000000000005291. (Under Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arfanakis K, Fleischman DA, Grisot G, Barth CM, Varentsova A, Morris MC, Barnes LL, Bennett DA. Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PloS one. 2013;8:e73107. doi: 10.1371/journal.pone.0073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettcher BM, Yaffe K, Boudreau RM, Neuhaus J, Aizenstein H, Ding J, Kritchevsky SB, Launer LJ, Liu Y, Satterfield S, Rosano C Health ABCs. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiology of aging. 2015;36:948–954. doi: 10.1016/j.neurobiolaging.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. J Neuroimmunol. 2008;193:183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Leung R, Proitsi P, Simmons A, Lunnon K, Guntert A, Kronenberg D, Pritchard M, Tsolaki M, Mecocci P, Kloszewska I, Vellas B, Soininen H, Wahlund LO, Lovestone S. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PloS one. 2013;8:e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 36.Vargas T, Ugalde C, Spuch C, Antequera D, Moran MJ, Martin MA, Ferrer I, Bermejo-Pareja F, Carro E. Abeta accumulation in choroid plexus is associated with mitochondrial-induced apoptosis. Neurobiol Aging. 2010;31:1569–1581. doi: 10.1016/j.neurobiolaging.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Da Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev. 2016;68:547–562. doi: 10.1016/j.neubiorev.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 39.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villeda SA, Wyss-Coray T. The circulatory systemic environment as a modulator of neurogenesis and brain aging. Autoimmunity reviews. 2013;12:674–677. doi: 10.1016/j.autrev.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Le Page A, Lamoureux J, Bourgade K, Frost EH, Pawelec G, Witkowski JM, Larbi A, Dupuis G, Fulop T. Polymorphonuclear Neutrophil Functions are Differentially Altered in Amnestic Mild Cognitive Impairment and Mild Alzheimer’s Disease Patients. J Alzheimers Dis. 2017;60:23–42. doi: 10.3233/JAD-170124. [DOI] [PubMed] [Google Scholar]
- 42.Maia LF, Kaeser SA, Reichwald J, Lambert M, Obermuller U, Schelle J, Odenthal J, Martus P, Staufenbiel M, Jucker M. Increased CSF Abeta during the very early phase of cerebral Abeta deposition in mouse models. EMBO Mol Med. 2015;7:895–903. doi: 10.15252/emmm.201505026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbeek MM, De Jong D, Kremer HP. Brain-specific proteins in cerebrospinal fluid for the diagnosis of neurodegenerative diseases. Ann Clin Biochem. 2003;40:25–40. doi: 10.1258/000456303321016141. [DOI] [PubMed] [Google Scholar]
- 45.Zemlan FP, Rosenberg WS, Luebbe PA, Campbell TA, Dean GE, Weiner NE, Cohen JA, Rudick RA, Woo D. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem. 1999;72:741–750. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, Lamb BT, Bhaskar K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA. Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 50.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 51.Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33:1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci. 2008;63:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, Kramer JH. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26:103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.