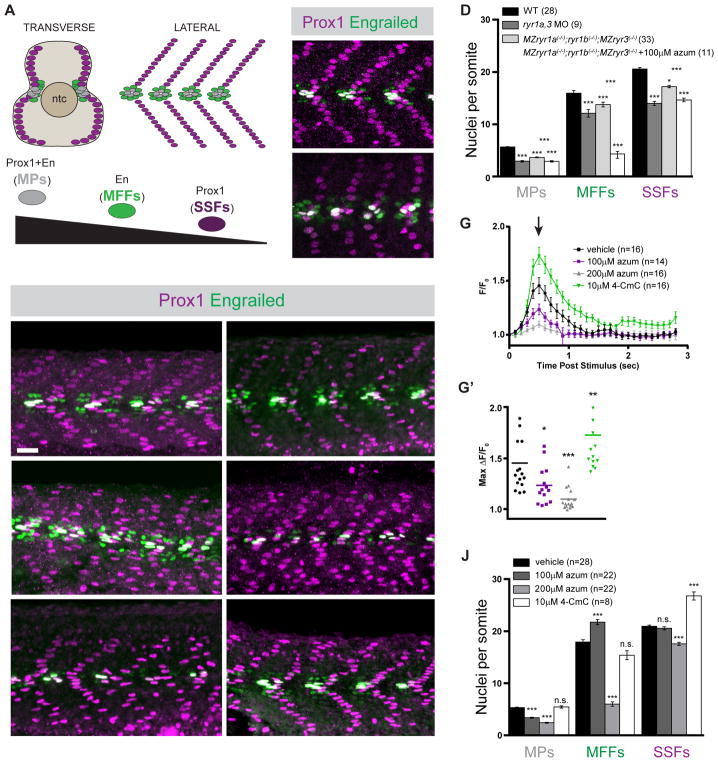
Figure 1.
Formation of Shh-dependent muscle requires RyR function. (A) Schematic illustration of the nuclei of Shh-dependent muscle cells in the somites of 26hpf zebrafish embryos, presented relative to the notochord (ntc) in transverse section and in a superficial lateral view (anterior to left). Nuclei of different muscle types are color-coded: Slow Muscle Pioneer Cells (MPs, grey), Medial Fast Fibers (MFFs, green), and Superficial Slow Fibers (SSFs, magenta). (B, C, E, F, H, I, K, L) Shh-dependent muscle fiber nuclei, marked by Prox1 (magenta) and En (green) expression, were visualized by immunostaining 26hpf embryos: (B) wildtype (WT), (C) ryr1a and ryr3 MO-injected (ryr1a,3 MO), (E) vehicle-treated (0.5% DMSO) WT, (F) MZryr1a(−/−);ryr1b(−/−);MZryr3(−/−) mutant, (H and I) azumolene-treated WT, (K) azumolene-treated MZryr1a(−/−);ryr1b(−/−);MZryr3(−/−) mutant, and (L) 4-CmC-treated WT (supernumerary Prox1+ SSF nuclei are indicated with arrowheads). All nuclei co-expressing En and Prox1 were considered MPs. Scale bar indicates 25 μm. (D and J) Quantification of distinct muscle cell types per somite. Data are represented as mean ±SEM. As numbers of nuclei in WT and DMSO-treated embryos did not differ significantly, comparisons were made to control vehicle-treated embryos unless otherwise indicated. (G) Traces of GCaMP fluorescence in electrically stimulated muscle of 24hpf Tg(act2b:GCaMP6f) embryos incubated from 6 to 24hpf in indicated solutions. Data are represented as mean ±SEM. The maximum change in fluorescence from baseline (arrow in G) is shown for each recorded embryo in (G′) with horizontal lines representing means. See also Figure S2.