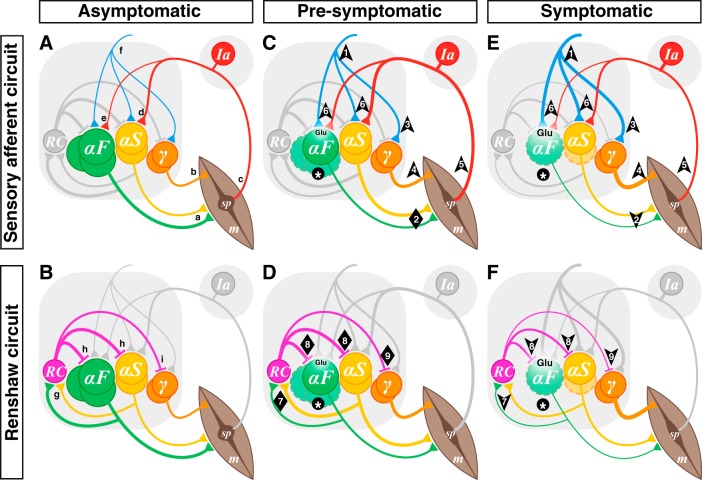
Fig. 1.
Hypothesis: α-motoneuron (α-MN) death leads to microcircuit imbalance and disease progression. Sensory afferent circuits (A, C, E) and Renshaw circuits (B, D, F) in asymptomatic (A, B), presymptomatic (C, D), and symptomatic (E, F) amyotrophic lateral sclerosis (ALS). A and B: normal spinal MN circuits. MN pools comprise multiple types: α-MNs can be defined by the extrafusal muscle fiber (m) types they innervate as either fast (αF; FF and FR types depicted together) or slow (αS) (a). γ-MNs innervate muscle spindles (sp; b), which convey length and velocity information back to α-MNs primarily via group Ia afferents (c), which form monosynaptic connexions with α-MNs (d, e). During most movement, α- and γ-MNs are coactivated by spinal and supraspinal neurons (f). α-MNs also innervate Renshaw cells (RC; g), which in turn inhibit both α- (h) and γ-MNs (i). C and D: in presymptomatic ALS, α-MNs (F-type) become dysfunctional and start to die (*), but γ-MNs are preserved. Homeostatic mechanisms include increased input to α-MNs from spinal and supraspinal circuits (1) to ensure that force production is preserved (2). Thus the input to the coactivated γ-MNs would also increase (3), leading to increased intrafusal fiber contraction (4) out of proportion to extrafusal fibers. This α-γ imbalance would result in an increase in spindle afferent input to α-MNs (6). The increasing glutamatergic (Glu) excitation from these inputs would initially maintain the homeostatic response despite a reduction of activity of fast high-force-producing muscle fibers. In addition, the loss of α-MNs (particularly type F; *) would concomitantly lead to a reduction of output from MN pools to RCs, initially compensated by increased α-MN activity (particularly type S; 7). Thus Renshaw inhibition would at first be maintained in all MN types (8, 9). Together, these processes would lead to increased glutamatergic excitation of vulnerable α-MNs and, hence, excitotoxicity. E and F: in symptomatic stages, type F α-MNs continue to die and type S α-MNs start to degenerate and ultimately die at later stages of the disease (*), but γ-MNs are completely spared. The processes that started in presymptomatic stages would continue, there would be runaway from homeostatic processes, and further excitotoxicity would lead to disease progression. It would no longer be possible to maintain muscle contraction (2), compounding the α-γ imbalance (2 and 4, 5), and the resulting loss of input to RCs (7) would reduce Renshaw inhibition of α-MNs (8) and also diminish γ-MN inhibition (9), thereby contributing to increased excitation of remaining α-MNs but a further imbalance of α-γ output. Note that the thickness of each line represents the integral of synaptic transmission over the number of synaptic contacts on the target cells. In the interest of simplicity and clarity, static and dynamic γ-MNs are represented as a single population, group II sensory afferents are not shown, and MN types are represented as distinct groups, although they are intermingled in each MN pool. Arrows indicate direction of change, and diamonds indicate no net change.