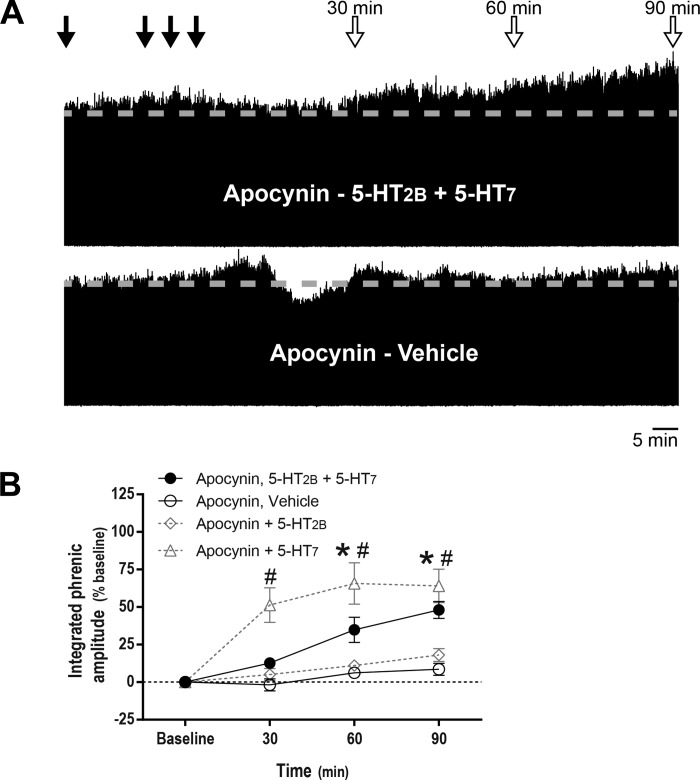
Fig. 3.
NADPH oxidase inhibitor apocynin restores phrenic motor facilitation (pMF) after concurrent serotonin 2B receptor (5-HT2B) and serotonin 7 (5-HT7) receptor activation. A: representative traces of compressed, integrated phrenic nerve burst amplitude at baseline and during and after intrathecal drug injections. Gray dashed line in each trace marks baseline amplitude of phrenic nerve activity. Top closed arrows depict intrathecal apocynin (600 μM, 12 μl) injections followed by episodic intrathecal drug injections; top open arrows depict 30-, 60-, and 90-min blood samples. B: average (± SE) values from baseline to 90 min after episodic intrathecal drug injections (expressed as %baseline). Lines are from rats pretreated with intrathecal apocynin injections followed by either 5-HT2B (BW 723C86 hydrochloride; 1 mM) plus 5-HT7 receptor agonists (AS-19; 10 μM, 3 × 6 μl, n = 8), or vehicle (3 × 6 μl, n = 6). In apocynin-pretreated rats, integrated phrenic nerve burst amplitude was significantly higher than baseline at 60 and 90 min post-5-HT2B and 5-HT7 receptor activation (*), and integrated phrenic nerve burst amplitude was also significantly higher than in vehicle-pretreated rats at 30, 60 and 90 min post-5-HT2B and 5-HT7 receptor activation (#). Although 5-HT7-dependent pMF was not affected by apocynin pretreatment (3 × 6 μl, n = 3), 5-HT2B-dependent pMF was blunted (3 × 6 μl; n = 5). Differences were considered significant at P < 0.05. Mixed, two-way ANOVA with repeated measures for time was used to detect overall differences between and within groups (interaction between time and groups was included in the model; F, 7.751; P < 0.001; df, 9), followed by Tukey's post hoc test.