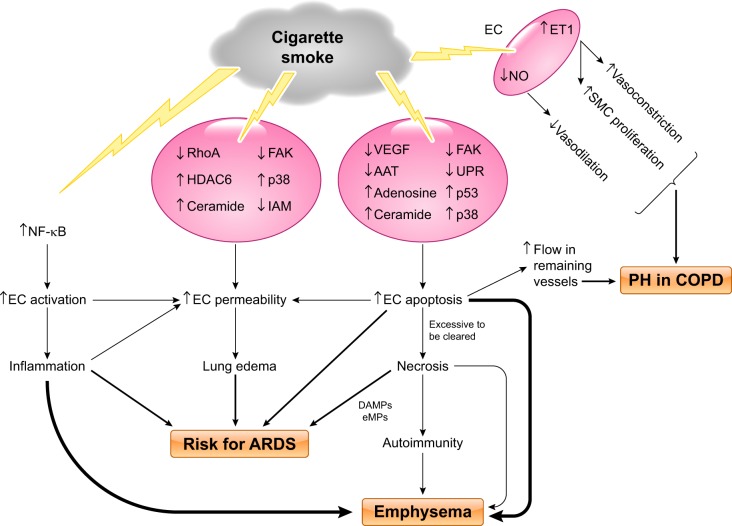
Fig. 4.
Proposed model of cigarette smoke-induced pulmonary endothelial cell injury. Cigarette smoke (CS) exposure causes lung endothelial cell (EC) activation, resulting in inflammation, via activation of NF-κB signaling; CS exposure directly increases EC permeability likely through multiple signaling pathways, including inhibition of RhoA, focal adhesion kinase (FAK), and intercellular adhesion molecules (IAM) and activation/upregulation of histone deacetylase-6 (HDAC6), p38, and ceramide; CS exposure also causes EC apoptosis through signaling pathways involving downregulation of VEGF, FAK, α1-antitrypsin (AAT), and unfolded protein response (UPR) and upregulation of ceramide, adenosine, p38, and p53. Excessive EC apoptosis can lead to necrosis and autoimmunity because of release of mitochondrial damage-associated molecular patterns (DAMPs), endothelial microparticles (eMPs), and other cellular contents. CS-induced increases in EC activation, permeability, apoptosis, and necrosis collectively contribute to increased risk of development of acute respiratory distress syndrome (ARDS). CS-induced lung inflammation, EC apoptosis and necrosis, and autoimmunity cause emphysema. CS exposure increases EC endothelin (ET)-1 levels, leading to increased smooth muscle cell (SMC) proliferation and vasoconstriction. CS exposure also decreases vasodilation because of reduction in EC NO. All these changes, in combination with increased blood flow in remaining vessels due to EC apoptosis and loss of vessels, cause vascular remodeling and pulmonary hypertension (PH) in chronic obstructive pulmonary disease (COPD) patients.