Abstract
Past animal and human studies robustly report that the cholinergic system plays an essential role in both top-down and bottom-up attentional control, as well as other aspects of cognition (see Ballinger et al., 2016 for a recent review). However, current understanding of how two major cholinergic pathways in the human brain (the basal forebrain-cortical pathway, and the brainstem pedunculopontine-thalamic pathway) contribute to specific cognitive functions remains somewhat limited. To address this issue, we examine how individual variation in the integrity of striatal-dopaminergic, thalamic-cholinergic, and cortical-cholinergic pathways (measured using Positron Emission Tomography in patients with Parkinson’s disease) was associated with individual variation in the initial goal-directed focus of attention, the ability to sustain attentional performance over time, and the ability to avoid distraction from a highly-salient, but irrelevant, environmental stimulus. Compared to healthy controls, PD patients performed similarly in the precision of attention-dependent judgments of duration, and in sustaining attention over time. However, PD patients’ performance was strikingly more impaired by the distractor. More critically, regression analyses indicated that only cortical-cholinergic integrity, not thalamic-cholinergic or striatal-dopaminergic integrity, made a specific contribution to the ability to resist distraction after controlling for the other variables. These results demonstrate that the basal forebrain cortical cholinergic system serves a specific role in executing top-down control to resist external distraction.
Introduction
Concepts of the brain’s cholinergic system and its role in cognitive function have changed dramatically in the last 20 years. Once viewed as a diffuse, “volume transmission” system primarily involved in regulating general states such as arousal and sleep/wake cycles (Perry et al., 1999), it is now understood to also have spatially and temporally specific organization and functions (see Ballinger et al., 2016, Lee & Dan, 2012; Zaborsky et al., 2015a, 2015b for review). However, the ability to link these systems-neuroscience discoveries about cholinergic neuroanatomy and transmission-receptor dynamics to specific cognitive functions has been restricted, in part because of the limitations inherent in animal tests of cognition and the degree to which these translate to human cognition. This is especially the case for higher-level functions such as cognitive control. The present study takes a step towards bridging that gap by examining the degree to which the integrity of the basal forebrain-cortical cholinergic system is related to specific aspects of controlled attention in patients with Parkinson’s disease (PD).
Just as concepts of the cholinergic system have become more sophisticated and complex, so too has understanding of neurodegeneration in PD. In addition to the dopaminergic declines that are the hallmark of the disease, there is often degeneration along other neuromodulator pathways, including cortical and thalamic cholinergic subsystems (Bohnen and Albin, 2011; Dunois et al., 1983; Müller and Bohnen, 2013; Perry et al., 1985; Pillon et al., 1989). These patterns of degeneration along defined neuromodulator pathways make studies of patients with PD (and other neurodegenerative disorders) an important complement to genetic and drug studies, which typically have effects throughout the brain, and especially in the case of drug manipulations, often alter the system in artificial ways that do not simply mimic an overall increase or decrease in activity.
While patient studies have their own limitations, they may help to identify the links between neuromodulator pathways and cognitive function more precisely. In addition to comparing patients’ results to those of healthy controls, one can ask how well individual differences in the location and degree of neurodegeneration across patients predict individual differences in the degree to which they are impaired in specific cognitive operations.
We recently used this approach to demonstrate that thalamic cholinergic innervation plays an important role in “bottom-up”, stimulus-driven attention and target detection (Kim et al., 2017). PD patients who had previously undergone PET scanning to assess the integrity of dopaminergic and cholinergic pathways were tested in a simple signal-detection paradigm with a perceptual challenge (rapidly changing, “flashing” background). Categorical analyses showed that only those PD patients who were classified as low cholinergic (activity below the normal range) had impaired signal detection relative to healthy controls. More incisively, regression analyses modeling the contributions of caudate-dopaminergic, cortical-cholinergic, and thalamic-cholinergic pathways showed that only the thalamic pathway made an independent contribution to signal detection after controlling for age and other variables.
In the present study, we use this method to test the hypothesis that the basal forebrain-cortical cholinergic system plays a critical role in “top-down”, goal-driven control, especially the ability to resist external distractors. The dual-syndrome hypothesis of cognitive impairment in PD (Kehagia et al., 2013) heuristically links executive control declines in PD to frontal-striatal dopaminergic degeneration, and cholinergic degeneration to PD dementia. However, it does acknowledge a cholinergic contribution to cognitive control, and there is mounting evidence to support this role. It has long been known that anti-cholinergic medications cause impairment on working memory tests (e.g., Dubois et al., 1987; 1990). More recently, reduced integrity of cortical cholinergic pathways as measured by PET has been linked to reduced performance on working memory tests such as digit span, and less robustly to executive-function tests such as Stroop and the Trail-Making Tests (Bohnen et al., 2006).
Most of the evidence linking cholinergic deficits to cognitive deficits in PD comes from studies that have used standardized neuropsychological batteries. The advantages of using such batteries include their reliability and the relative ease of comparing results across studies, including studies of other patient populations. Broadly speaking, the results of such studies link basal forebrain-cortical cholinergic pathway decline to cognitive declines, whereas the pedunculopontine-thalamic cholinergic pathway may be more related to sensory processing and integration (Müller et al., 2013). However, the disadvantage of such batteries is that their broad nature limits the conclusions that can be made about more specific cognitive processes.
The present study therefore tested patients with varying levels of cholinergic denervation in the Continuous Temporal Expectancy task (CTET, O’Connell et al., 2009) with video distractor (Berry et al., 2014a, 2014b), which allows simultaneous assessment of multiple dimensions of attention. The CTET requires participants to monitor a stream of stimuli that typically change orientation after a standard time interval; the target is visually identical to the standards (nontargets) and only differs from them in taking slightly longer to change. Target detection thus relies heavily on the top-down, goal-directed attentional focus on internal representations of time, with little or no “bottom-up” support from perceptual salience (see Grondin, 2010; Meck & Benson, 2002; Zakay & Block, 1997 for reviews). It is difficult to maintain this level of focus, and performance declines significantly in only a few minutes. External distraction is manipulated via a laptop placed next to the main task computer that is either silent and displaying a gray screen (no-distractor condition) or playing a series of video clips (distractor condition).
The video CTET thus indexes the goal-directed focus of attention (target detection), sustained attention (the ability to maintain performance over the duration of each “run”) and distractibility (changes in performance between the no-distractor vs distractor conditions). Furthermore, these indices have been shown to be independent and dissociable along dimensions including stimulus modality, age group, genetic group, and monetary incentive. In healthy populations, the different indices of the CTET also show differential correlations with EEG measures and self-report measures of mind-wandering, boredom, and distractibility during the task and in everyday life (Berry et al., 2014a, 2014b; Lin, Berry, and Lustig, in prep.; O’Connell et al., 2009). Of particular relevance to the present study, individuals with a genetic polymorphism thought to limit cholinergic function report greater distractibility in everyday life and on the CTET show a specific deficit in the ability to resist distraction, whereas target detection and sustained attention remain intact (Berry et al., 2014b).
Cortical cholinergic function may play an especially important role in the degree to which behavior is governed by “top-down”, goal-driven attention versus “bottom-up”, stimulus-driven attention (see discussions by Lustig & Sarter, 2016; Sarter et al., 2016). We therefore tested the hypothesis that cholinergic denervation in PD patients would be specifically associated with an increased vulnerability to distraction from the videos, which have a great deal of bottom-up stimulus salience but are irrelevant to the primary task.
We first compared patients to healthy control (HC) participants to provide an overall picture of their clinical status. However, duration judgments rely heavily on intact dopaminergic function (Ivry and Spencer, 2003; Meck 1986; 1996; Rammsayer 1993), and thus patients would be expected to show a generally lower target detection rate than HC (since target detection in this case depends on accurate duration judgements). Our primary hypothesis is that cortical cholinergic denervation should show a unique ability to predict vulnerability to distraction, but be unrelated to overall target detection or sustained attention over time. Therefore, the more critical test of our hypothesis is in the regression analyses conducted on the PD patient data: Cortical cholinergic denervation should show a unique ability to predict vulnerability to distraction, but be unrelated to overall target detection or sustained attention over time.
Materials and Methods
Participants
The majority of participants reported here also participated in the study reported by Kim et al. (2017); investigators interested in the demographic or PET data for meta-analyses may contact the authors for details in order to ensure independent estimates on these variables. To avoid confusion, with permission of the editors we largely reproduce the text describing the overall structure of the data collection and analysis procedures. Those readers familiar with our earlier paper may wish to focus on the descriptions of the video CTET measures that are of central interest to the present hypothesis.
All experimental procedures were approved by the University of Michigan’s Institutional Review Board, and were fully described to the participants before they consented to take part in the study. PD patients were recruited from an existing pool who had previously undergone cholinergic and dopaminergic PET scanning within one year of the present study (see brief description below; for further details see supplemental methods or Bohnen et al., 2012). Healthy control (HC) participants were recruited from the Ann Arbor community to be age-, gender-, and education-matched to the PD patients and did not undergo PET scanning. PD patients were compensated for their time at a rate of $25/hour and HC participants were compensated at a rate of $10-12/hour (the payment rate went up for HC during data collection).
Inclusion criteria for the present study included the absence of a history of seizures, severe brain injury, and neurological disorders other than PD. The Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) was used to screen for dementia. The Extended Range Vocabulary Test Version 3 (ERVT; Educational Testing Services, 1976) was used to screen out participants who might be unable or unwilling to understand and follow instructions; all participants scored above the minimum threshold of 9/48 correct responses. All participants were tested and screened for normal or corrected-to-normal vision and hearing.
A total of 20 PD patients and 20 healthy age-, gender-, and education-matched controls (healthy controls, HC) completed the study. Age and education matches were within a 3-year margin of error within a pair. Three patient-control pairs were eliminated from final analysis due to outlying (ceiling/floor) CTET performance that distorted the results: One patient showed ceiling performance across all conditions and also reported being an extraordinary case in attention skill due to prior training as a Morse-code decoder. Two other patients showed a pronounced reversed distractor effect, falling outside 1.5 standard deviations from the group average. Thus, final analyses included 17 PD patients (5 female; mean age = 65.9; SD = 10.18, age range 52-85) and their healthy controls (± 3 years; mean age = 66.5; SD = 9.55; age range 53-84).
On average, motor symptom duration of PD patients was 5.1 years (SD = 4.0 yrs; range, 1-14 yrs), and the median Hoehn and Yahr PD severity score, assessed in the dopaminergic “off” state, was 2.0 (SD = 0.40; range, 1.5-3.0; 1-5 scale, scores of 4 or more indicate severe disability; median reported as it is an ordinal scale; Hoehn and Yahr, 1967). All patients except one were on dopaminergic treatment (average levodopa equivalent daily dose (LEDD; Tomlinson et al., 2010) for those on dopaminergic treatment was 588 mg, range: 100–1596 mg). No patient was taking any cholinergic or anti-cholinergic medications. Two patients were also being treated for anxiety, 1 for depression, 2 for comorbid anxiety and depression, and 1 for comorbid anxiety, depression, and panic disorder. We did not exclude these patients because depression and/or anxiety are frequently co-morbid with PD, occurring in 40-50% of patients (Cummings, 1992; Tandberg et al., 1996), and thus can be considered typical of the disorder. One HC reported a previous diagnosis of depression but was not currently in treatment.
Participants also completed standardized self-report and neuropsychological tests evaluating the ability to maintain independent function in everyday life and affective, cognitive, and motor function. The measures included the Instrumental Activities of Daily Living scale (IADL; Lawton and Brody, 1969), Apathy Evaluation Scale (AES; Glenn, 2005), Beck Depression Inventory II (BDI-II, Beck et al., 1961), and Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS; copyright: Movement Disorder Society; Goetz et al., 2007).
Continuous Temporal Expectancy Task (CTET) with video distractor
Both the CTET and the distractor videos were presented on HP laptops (Windows7) with a 34.5×19.5 cm LCD screen (1024×768 screen resolution, 60Hz refreshing rate). The laptop used to present the CTET was placed in front of the participants at a 57 cm distance, and the laptop used to present the distractor videos was placed on the left at a 45 degree angle from the task laptop (Figure 1(B)). E-prime software (Psychology Software tools; http://www.pstnet.com/eprime.cfm; version 2.0) was used for CTET stimuli presentation and response recording. Participants wore headphones connected to the laptop presenting the distractor videos, and responded to the CTET using the keyboard on the laptop used to present that task.
Figure 1. CTET with video distractor manipulation.
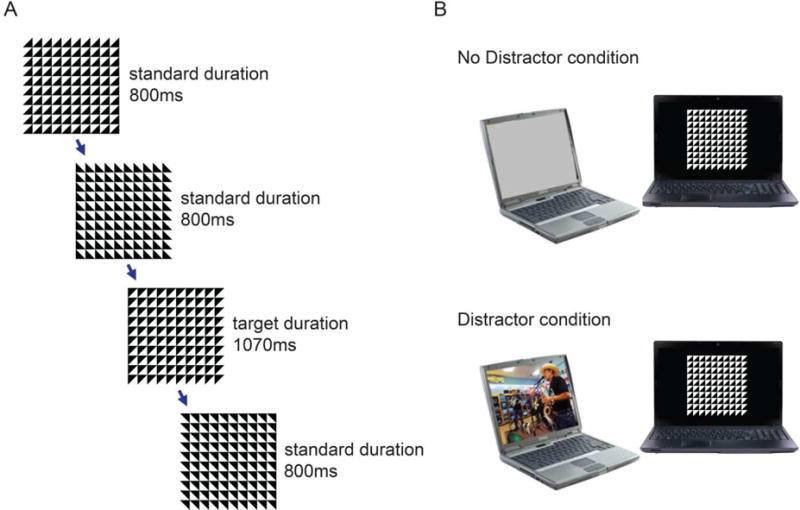
As shown in (A) each trial consisted of a black and white grid made up of squares divided into triangles. At the end of the trial, the triangles rotated (90,180, or 270 degrees, chosen randomly) to start the next trial. The participant’s task was to press the spacebar when they realized that the grid had taken longer than usual (1070 ms rather than the standard 800 ms) to rotate. (B) The distractor manipulation was implemented using a laptop oriented 32° to the left of the main task computer. In the No Distractor condition, the laptop was silent and displayed a gray screen. In the Distractor condition, it played video clips with sound.
On each CTET trial, participants were presented with a black and white 10×10 grid of square tiles (1.27 cm2 each) divided diagonally into black and white halves. On standard trials, the grid randomly changed orientation (90, 180, or 270°) after 800 ms; on target trials it rotated after 1070 ms. (Figure 1(A)). There was a 20 ms long empty grey screen after each rotation. Participants were instructed to press the spacebar on the laptop keyboard as soon as they detected the target. Responses made during the target display and the following 2480 ms were counted as hits, other responses were counted as false alarms.
Data collection occurred during 10 four-minute long runs, 5 in the No Distractor condition and 5 in the Distractor condition, interleaved. There were 24 targets per run, pseudo-randomly intermixed with 4-8 target trials presented per minute and 7-14 standard-duration stimuli presented between each target stimulus. Run order and assignment of distractor condition (No Distractor vs Distractor) to odd vs even runs was counterbalanced across subjects. In the No Distractor condition, the laptop used for video presentation was silent and displayed a gray screen. In the Distractor condition, the laptop played a series of 30 second video clips from various sources (e.g., cartoons, movies, sports) with sound presented via headphones. Each of the four-minute distractor series consisted of a unique set of video clips; order of clips remained constant within each series and the order of series assignment to Distractor run was counterbalanced across participants. None of the videos contained music or other obviously rhythmic content, or overtly violent or sexual content.
Participants first received verbal instruction on the task followed by practice. By default, 6 short blocks of practice were given. A practice block was approximately 30 seconds long, and always contained 3 targets. In the very first practice block, the rotation delay of the targets was exaggerated (1600ms; 800ms longer than non-target trials) in order to make it clear to the participants what they should be looking for. From the second practice block on, the delay was the same as in the experimental blocks (1070ms; 270 ms longer than non-target trials). Participants had to detect all 3 targets in at least one of the five blocks using the 1070 ms target before moving to the experimental trials. Only two participants in each group failed to reach criterion in the first round of practice, they completed another 5 blocks of practice using the 1070 ms target, and met criterion within this round.
As described above (see Introduction), three specific aspects of sustained attention were assessed using this task. Goal-directed focus of attention is operationally defined here as the capability to detect the target duration. To minimize confounds from distractor and/or time-on-task effects, or potential interactions, we used the hit rate during the first minute in the No Distractor condition. Sustained attention (or, time-on-task effect) was measured as the hit rate change slope in No Distractor condition (in order to avoid confounds from potential interaction effect from distraction). The hit rate difference between No Distractor and Distractor conditions during the first minute served as the measure of distractibility (distractor effect), to avoid confounds from the fatigue/time effect. Importantly, these measures are fairly uncorrelated in healthy subjects (all | r | < .5, all p > .05; see table 2 for individual r and p values), affirming that these are dissociable dimensions of sustained attention function.
Table 2. Correlations between age, depressions score (BDI), the behavioral measures, and the PET measures.
Initial focus = hit rate in minute1, attentional decline = hit rate decrease over time, distractor effect = hit rate difference between the no distractor vs. distractor condition in minute 1.
| individual characteristics | video CTET | cholinergic PET | dopaminergic PET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| age | BDI | initial focus | attention al decline | distracto r effect | thalamic k3 | cortical k3 | putamen DVR | caudate DVR | |||
| individual characte ristics | age | r | 1.00 | −.37 | −.20 | −.18 | .46 | −.02 | −.34 | −.01 | −.33 |
| p | .138 | .432 | .481 | .065 | .946 | .181 | .968 | .202 | |||
| BDI | r | −.37 | 1.00 | .20 | −.29 | −.20 | .24 | .50 | .08 | .34 | |
| p | .138 | .439 | .259 | .442 | .360 | .040 | .751 | .185 | |||
| video CTET | initial focus |
r | −.20 | .20 | 1.00 | .32 | −.06 | .10 | .13 | −.34 | −.27 |
| p | .432 | .439 | .213 | .819 | .713 | .622 | .187 | .285 | |||
| attention al decline |
r | −.18 | −.29 | .32 | 1.00 | −.58 | −.19 | .19 | .00 | .04 | |
| p | .481 | .259 | .213 | .015 | .463 | .465 | .996 | .869 | |||
| distractor effect |
r | .46 | −.20 | −.06 | −.58 | 1.00 | −.01 | −.52 | −.30 | −.38 | |
| p | .065 | .442 | .819 | .015 | .967 | .031 | .237 | .129 | |||
| choliner gic PET | thalamic k3 |
r | −.02 | .24 | .10 | −.19 | −.01 | 1.00 | .67 | .29 | .37 |
| p | .946 | .360 | .713 | .463 | .967 | .003 | .256 | .149 | |||
| cortical k3 |
r | −.34 | .50 | .13 | .19 | −.52 | .67 | 1.00 | .33 | .50 | |
| p | .181 | .040 | .622 | .465 | .031 | .003 | .191 | .041 | |||
| dopamin ergic PET | putamen DVR |
r | −.01 | .08 | −.34 | .00 | −.30 | .29 | .33 | 1.00 | .75 |
| p | .968 | .751 | .187 | .996 | .237 | .256 | .191 | .001 | |||
| caudate DVR |
r | −.33 | .34 | −.27 | .04 | −.38 | .37 | .50 | .75 | 1.00 | |
| p | .202 | .185 | .285 | .869 | .129 | .149 | .041 | .001 | |||
Memory for the video distractor and self-reported attentional function during the task
To assess the degree to which the videos captured attention and drew it away from the CTET, we administered a short surprise quiz (15 items, multiple choice) testing memory for the content of the distractor videos.
Participants were next asked to rate their experience during the task. This consisted of five statements asking participants to rate the degree to which they identified with each statement on a scale from 1 to 5. Questions 1, 2, and 4 measured mind-wandering, question 3 measured boredom, and question 5 measured distractibility. Our previous studies have shown that questions 3-5 show the strongest relationships with “trait” PAC scores and performance on different indices of the video CTET, thus as in those previous studies we again focus our analyses on these questions as the “state” measures of boredom, mind-wandering, and distractibility.
PET
For the PD patients, Positron Emission Tomography (PET) scan data on dopaminergic and cholinergic nerve terminal integrity were obtained from previous studies (Bohnen et al., 2012). (HC were not scanned, but were chosen to be closely matched to patients in age, gender, and education.) Patients came in for dopaminergic PET scanning in the dopaminergic off-state, i.e. after abstaining from dopaminergic drugs overnight. The PET scans were obtained prior to the behavioral testing session (median .84 yrs; SD = .59 yrs).
The integrity of dopaminergic nigrostriatal nerve terminals was measured with [11C]dihydrotetrabenazine (DTBZ), a vesicular mono-amine transporter type 2 analogue (VMAT2; see Bohnen et al., 2012 for details on DTBZ preparation, injection, and scanning parameters). The primary outcome parameter is DTBZ distribution volume ratio (DVR, Bohnen et al., 2009). Greater DVR indicates better dopaminergic terminal function. DTBZ DVR was measured for caudate and putamen. Mean DVR values for PD patients in the present study were M = 2.3002, SD = .4541 for caudate, M = 1.9273, SD = .3132 for putamen, M = 2.0516, SD = .3375 for whole striatum (average over putamen and caudate). Values reported from a larger sample in Bohnen et al. (2012) were striatum (average over putamen and caudate) DVR M=1.93, SD=0.27 for PD patients (N = 101) and M=3.03, SD=0.31 for HC (N = 29).
Cholinergic function was estimated using radio-labeled acetylcholine analogue [11C]methyl-4-piperidinyl propionate (PMP) PET, which measures acetylcholinesterase (AChE) activity. PMP PET scans were performed in the dopaminergic medication ‘on’ state. Details on PMP preparation, injection, and scanning parameters have been described previously (Bohnen et al., 2012). The primary outcome parameter is AChE hydrolysis rate (k3; min-1), with a higher k3 indicating higher cholinergic nerve terminal integrity. Although PMP PET is an indirect measure of cholinergic activity, it has been validated in both rodent and primate models (e.g, Kilbourn et al., 1996; Selden et al., 1998) as well as in humans (e.g., Kuhl et al., 1996b; 1999). For example, Kuhl et al. (1996a) showed that distribution of AChE activity traced using PMP is well correlated with postmortem histochemical distribution (Bohnen and Frey, 2007 for review). We are not aware of any evidence suggesting that dopaminergic therapy affects brain AChE hydrolysis rates. To the extent that dopaminergic therapy may affect cholinergic activity, its effects may be more likely to be on the availability of nicotinic or muscarinic receptors rather than AChE, given its long half-life of approximately 2.8 days (Wenthold et al., 1974).
AChE k3 was measured for the cortex and thalamus separately. Cortical measures are used to index cholinergic nerve terminal integrity of the basal forebrain (including the nucleus basalis of Meynert), whereas thalamic measures primarily (though not exclusively) reflect integrity in the brainstem pedunculopontine nucleus (Bohnen and Albin, 2011; Bohnen et al., 2012; see review by Varela, 2014). Mean k3 values for PD patients in the present study were cortical M = .0246, SD = .0034; thalamic M = .0541, SD = .0065. For comparison, the values reported by Bohnen et al. (2012) were PD (N = 101): cortical M = .0236, SD = .0027; thalamic M = .0542, SD = .0056), HC (N = 29): cortical M = .0263, SD = .0027; thalamic M = .0599, SD = .0074.
Procedure
At the beginning of the experimental session, participants first completed informed consent procedures and a health and demographic information questionnaire. Then they completed the CTET, followed by a surprise memory quiz on the contents of the video clips (distractors), the state PAC questionnaire, and another computerized task that was part of a different study (reported in Kim et al., 2017). The order of the two computerized tasks was counterbalanced across subjects. After completing the two computerized tasks, participants completed the ERVT, the Edinburgh handedness Inventory (Oldfield, 1971), and 36 items from the Imaginal Processes Inventory (IPI) questionnaire (Singer and Antrobus, 1970). The IPI items included the Poor Attentional Control (PAC) scale (Huba et al., 1982) and its subscales for boredom, mind- wandering, and distractibility (See Supplemental Material for details on PAC).
In a separate session, participants completed the IADL (Lawton and Brody, 1969), AES (Glenn, 2005), BDI-II (Beck et al., 1961), MoCA (Nasreddine et al., 2005), and MDS-UPDRS (Goetz et al., 2007). PD participants were additionally examined for Hoehn and Yahr severity scale by the neurologist (NB). Motor examination of the PD patients by the neurologist, which was performed in a separate session, was performed in the dopaminergic “off” state and included Hoehn and Yahr staging and UPDRS rating. One limitation of the study is that we do not have motor and cognitive scores collected both at the time of the PET scan and this behavioral session. This might have allowed us to assess changes in those scores that would add noise to the relationship between the PET values and behavioral performance. However, Lawson et al. (2016) did not find systematic changes in either motor or cognitive scores at either 18 or 36 months in patients with similar MoCA (26.2 vs 27.2) scores as in the current study. This suggests these scores likely stay stable during the time period in question here.
Statistical Analysis
Two-tailed independent sample t-tests were used to compare the HC and PD participants on demographic, motor, and self-report measures.
To provide a general picture of how PD patients compared to healthy controls, the video CTET results were first analyzed using a mixed-design ANOVA with group (PD, HC) as the between-subjects variable and time (minute 1, 2, 3, 4) and distractor condition (No Distractor, Distractor) as within-subject variables. As in previous studies using this task (Berry et al., 2014a; 2014b; O’Connell et al., 2009), false alarms were rare and generally uninformative and so analyses focus on hit rates. Full performance measures (hits, false alarms, d’, and bias) are reported in Supplemental Materials for the interested reader (Supplemental Table 1 and Supplemental Tables 6-8). Greenhouse-Geisser sphericity correction was applied if needed, in which case the corrected degrees of freedom (rounded to integers for easier reading), F, and p values are reported. Bivariate correlations were used to evaluate the relationships between the video CTET performance indices and the trait and state self-report measures of attentional control in each group. We report a standard set of correlation analyses across all of our papers using the video CTET, PAC, and post-task questionnaire measures (Supplemental Tables 2 & 3), in order to facilitate meta-analyses and tests of replication. Readers interested in this information are referred to the Supplemental Materials; analyses reported in the main text focus on those correlations relevant to our hypotheses.
Our central hypothesis was that cortical cholinergic denervation should show a unique relation to distractor vulnerability in the CTET, over and above the influence of other variables. First, bivariate correlation analyses were used to provide an initial picture of the relationships between the neural measures and our measures for three different dimensions of attention – initial performance (hit rate in minute1), performance decline over time (the slope of the hit rate changes over minute 1 to minute 4 in the absence of distractor), and the distractor effect (hit rate difference in no distractor vs. distractor condition from minute 1). Then hierarchical multiple regression was used to evaluate how much of the variance in performance was predicted by specific neural measures. In order to maintain comparability across studies (Kim et al., 2017; Kim et al., in prep.) we first report the model with all of the PET measures entered together. To more powerfully demonstrate the impact of the cortical k3 predictor, including the possibility of suppression effects between the thalamic and cortical measures, we also show the model with cortical k3 added as a final step.
As effect sizes, we report Cohen’s d for t-tests, generalized eta squared (η2G, Olejnik & Algina, 2003) for repeated measures ANOVAs, Pearson’s r for bivariate correlations, and standardized beta coefficient for multiple regression. Generalized eta squared typically provides smaller values than the eta squared (η2) or partial eta squared (η2p) values that are automatically generated by SPSS and other statistical packages (and which are thus more frequently reported), but is considered preferable as it as it allows comparison of effect sizes across studies, including across between-subjects and within-subjects designs (Bakeman 2005; Fritz et al., 2012). G*power software (v 3.1., Faul et al., 2007; 2009) was used to estimate power for the multiple regression analyses. Statistical analyses were conducted using SPSS (version 21) and R (version 3.1.1).
Results
Participant Groups
Table 1 provides comparisons for the demographic information, neuropsychological test results, self-reported attentional control, and overall performance of the PD and HC groups. The PD and HC groups were equivalent in age, years of education, verbal ability (ERVT), general cognitive function (MoCA), instrumental activities of daily living (IADL), and apathy evaluation scale (AES) scores. PD patients had significantly higher scores on the motor impairment measure (MDS-UPDRS III), depression score (BDI), and the Poor Attentional Control (PAC) scale (see Supplemental Material for PAC). As described above, the higher BDI scores in PD are expected, as mild to moderate depressive symptoms occur in 40-50% of PD patients (Cummings, 1992; Tandberg et al., 1996), and we therefore did not exclude participants on that basis. Based on the Edinburgh Handedness Inventory, one participant was left-handed (HC), 26 right-handed (13 PD, 13 HC), and the remaining 7 were ambidextrous (3 HC, 4PD).
Table 1. HC and PD groups.
Demographics, general cognitive functions (MoCA), affective states (AES, BDI), motor impairment (MDS-UPDRS III), recognition memory test for the video distractors, and video CTET performance in PD patients and HC (t and p values for the IADL and MDS_UPDRS scores are corrected for violation of equal variances).
| HC | PD | t | p | Cohe n’s d | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Age (years) | 66.5 | 9.5 | 65.9 | 10.2 | .2 | .863 | .06 |
| Education (years) | 16.8 | 2.0 | 16.7 | 2.4 | .0 | .969 | .05 |
| Extended Range Vocabulary Test | 26.7 | 8.0 | 26.5 | 8.1 | .1 | .941 | .03 |
| Montreal Cognitive Assessment | 27.2 | 1.9 | 26.7 | 2.3 | .7 | .517 | .24 |
| Instrumental Activities of Daily Living | 7.9 | 0.3 | 8.0 | 0.0 | −1.5 | .163 | N/A |
| Apathy Evaluation Scale | 24.7 | 6.4 | 25.9 | 8.2 | −.5 | .629 | .17 |
| Beck Depression Inventory | 4.4 | 3.6 | 8.9 | 4.7 | −3.1 | .004 | −1.11 |
| Motor UPDRS (MDS-UPDRS Part III) | 5.1 | 3.6 | 30.5 | 13.6 | −7.4 | ** | −2.63 |
| Video Quiz | 51.0 | 25.9 | 57.2 | 23.2 | −.7 | .463 | .26 |
| CTET initial attentional focus | .87 | .12 | .81 | .10 | 1.8 | .079 | .56 |
| CTET attentional decline | −.04 | .04 | −.04 | .04 | .1 | .911 | .00 |
| CTET distractor effect | .10 | .09 | .13 | .10 | −.9 | .380 | −.33 |
indicates p < .0005
CTET performance comparison between PD and HC
Figure 2 depicts the CTET performance data for both groups. Independent sample t-tests on the three CTET measures revealed only a marginal group difference in initial performance levels thought to reflect the ability to focus attention on internal representations of time (Table 1, t(32) = 1.81, p = .08, Cohen’s d = .56; there were no significant group differences in either the slope of the no-distractor condition (a measure of the decline of attention over time) or the distractor effect, both p > .5). To further characterize the effects, we analyzed the data (hit rates) using a 2 × 2 × 4 (group × distractor × time) ANOVA. For comparisons involving the effects of time, the linear contrast was used rather than the standard F value, consistent with testing the hypothesis of a systematic decrease in performance over time. We replicated the typical findings that both time on task (F (3, 96) = 32.45, p < .0005, η2G = .08) and the distractor (F (1, 32) = 58.92, p < .0005, η2G = .08) reduced performance, but did not interact, F < 1.
Figure 2. CTET performance with and without video distractor.
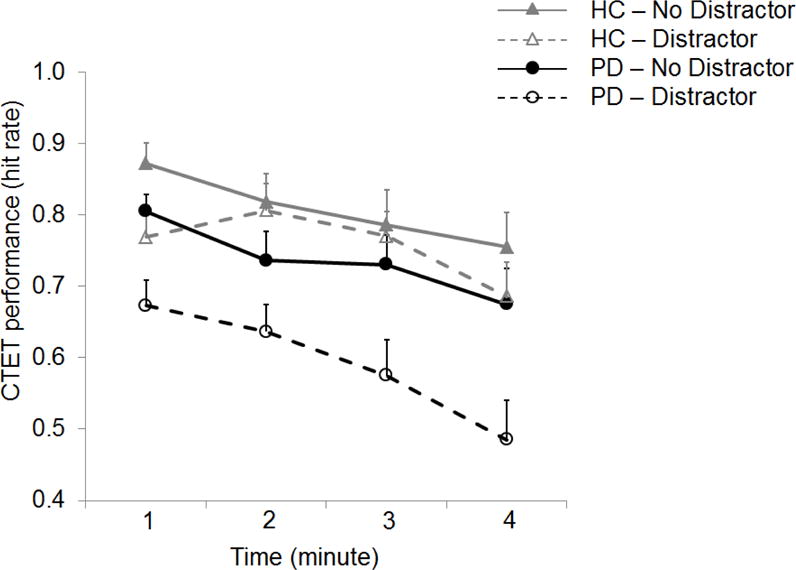
In the absence of external distraction (no distractor condition; filled markers and solid lines), decline of sustained attention did not differ between the groups. However, external distraction (distractor condition; open markers and dotted lines) impaired the performance more in PD than HC. Markers represent the average hit rates for each minute and error bars represent standard error of the mean.
Overall, HC showed better performance than PD (F(1, 32) = 5.37, p = .027, η2G = .12). Importantly, the distractor effect was significantly greater in PD than HC (distractor by group interaction; F (1, 32) = 13.85, p =.001, η2G = .02). In contrast, the two groups did not significantly differ in the degree to which performance declined over time (no time by group interaction; F (3, 96) = 1.72, p = .168, η2G = .005).
None of the higher-order interactions reached statistical significance, all p > .10. However, the HC showed an unusual pattern of less distraction at minutes 2 and 3. We have not seen such a pattern in any of our other datasets, including three separate samples of healthy older adults (Lin et al., in prep.), and so believe it is a chance finding. In the PD patients, there is some suggestion that the distractor effect may start to become larger at the last timepoint. A separate ANOVA conducted only on the PD patients did not find a significant time × distraction interaction, F(3, 48) = 1.46, p = .238, η2G =.010). Likewise a within-sample t-test comparing the slopes for the no-distractor and distractor conditions was not significant, t(16) = 1.42, p = .176, Cohen’s dz = .34. A G*Power analyses indicated that approximately 70 patients would be required to reliably (power = .80) detect an effect of this small-to-moderate size. Nonetheless, to avoid the possibility that either of these findings might lead to an exaggeration of the distractor effect in the PD, our analyses focus on the differences between the distractor and no distractor conditions in minute 1.
PD patients: Cortical cholinergic measures uniquely predict distractor effects
As noted above, the comparisons between the HC and PD patients and overall reports of demographic, health, and performance measures are described to provide a general clinical picture of our participants. However, our critical hypothesis concerned the relation between cortical cholinergic integrity and the distraction effect in the PD group. We next turn to these analyses.
Table 2 shows the first-level bivariate correlations (Pearson’s r) between the performance measures, the PET measures of cholinergic and dopaminergic integrity, and individual difference variables (age and depression score (BDI)) that might contribute to variance on the performance and PET measures. Neither the initial performance level thought to measure the focus of attention, nor performance decline over time (slope) correlated with age, BDI score, or any of the PET measures. In contrast, the distractor effect (hit rate difference between the no distractor vs. distractor condition in minute 1) showed moderate to strong correlations (absolute r values between .30 and .52) with age, cortical k3, putamen DVR, and caudate DVR. There were also moderate correlations between age and cortical k3 and caudate DVR (absolute r values .33-.34).
Because of the correlations between the potential predictor variables (age and the PET measures), we conducted hierarchical regression analyses to determine their unique contributions to the distractor effect. Although thalamic k3 did not correlate with the distractor effect or age, it was included as a predictor in the hierarchical regression model in order to allow comparisons with the results from our earlier findings (Kim et al., 2017). In all of the analyses reported here, the collinearity statistics were within acceptable ranges (tolerance values above .41; values above .10 are typically considered acceptable; all VIF values below 2.4; values below 10 are usually considered acceptable; Field, Miles, and Field 2012).
Our primary question was whether more severe cholinergic denervation might increase vulnerability to the distractor. Accordingly, we used the distractor effect as the criterion variable. To maintain comparability with Kim et al. (2017), we first tested the same regression model: As predictor variables, age was entered in the first step, followed by cortical k3, thalamic k3, and caudate DVR in a single step (See Table 3). Critically, in the final model, only cortical k3 was a significant predictor of the distractor effect over and above the other variables, with thalamic k3 being a marginally significant predictor in the opposite direction. Greater vulnerability to the distractor was associated with lower cortical k3 (b* = .77, t = −2.51, p = .027; See Figure 3) and higher thalamic k3 (b* = .56, t = 2.00, p = .068). Caudate DVR did not approach significance, t < 1.
Table 3. Hierarchical multiple linear regression model for distractor effects.
B, unstandardized coefficient; β, standardized coefficient
| coefficients | model statistics | power | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | R2 | Δ R2 | Δ F | sig. Δ F | Model Fit F | Model Fit p | 1−β | |
| step 1 model | .21 | .21 | 3.98 | .065 | 3.98 | .065 | .51 | ||||
| constant | −.15 | −1.05 | .309 | ||||||||
| age | .00 | .46 | 1.99 | .065 | |||||||
| step 2 model | .53 | .32 | 2.70 | .092 | 3.36 | .046 | .84 | ||||
| constant | .20 | .833 | .421 | ||||||||
| age | .00 | .15 | .681 | .509 | |||||||
| caudate DVR | −.03 | −.15 | −.658 | .523 | |||||||
| thalamic k3 | 8.25 | .56 | 2.00 | .068 | |||||||
| cortical k3 | −21.73 | −.77 | −2.51 | .027 | |||||||
Figure 3. Correlation between cortical k3 and the distractor effect after controlling for age thalamic k3, and caudate DVR.
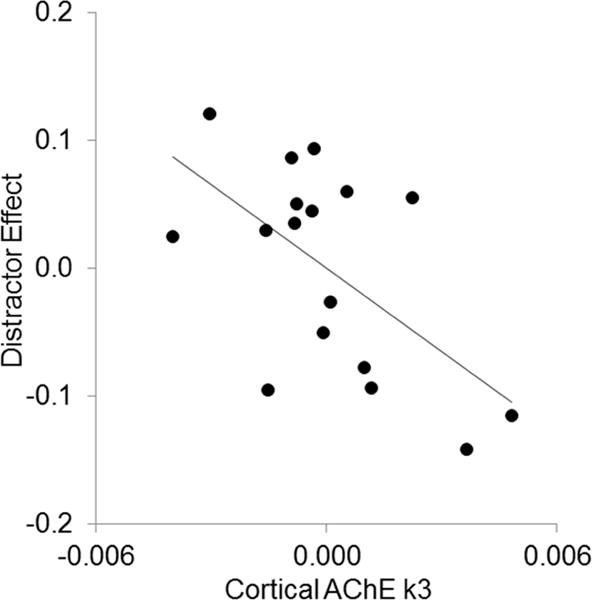
Lower cortical k3 levels were associated with a larger distractor effect (r = −.59, p = .013).
To highlight the unique contribution of cortical k3, Table 4 shows a similar regression model in which cortical k3 was entered into the model separately at the last step. Inclusion of cortical k3 in the model explained additional 25% of variance in the distractor effect (∆R2 = .25), significantly increasing the model fit (p = .027).
Table 4. Hierarchical multiple linear regression model for distractor effects with cortical k3 entered separately.
B, unstandardized coefficient; β, standardized coefficient
| coefficients | model statistics | power | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | R2 | Δ R2 | Δ F | sig. Δ F | Model Fit F | Model Fit p | 1−β | |
| step 1 model | .21 | .21 | 3.98 | .065 | 3.98 | .065 | .51 | ||||
| constant | −.15 | −1.05 | .309 | ||||||||
| age | .00 | .46 | 1.99 | .065 | |||||||
| step 2 model | .28 | .07 | .64 | .541 | 1.69 | .218 | .43 | ||||
| constant | −.03 | −.11 | .917 | ||||||||
| age | .00 | .36 | 1.44 | .174 | |||||||
| caudate DVR | −.06 | −.31 | −1.14 | .277 | |||||||
| thalamic k3 | 1.57 | .11 | .42 | .681 | |||||||
| step 3 model | .53 | .25 | 6.30 | .027 | 3.36 | .046 | .84 | ||||
| constant | .20 | .83 | .421 | ||||||||
| age | .00 | .15 | .68 | .509 | |||||||
| caudate DVR | −.03 | −.15 | −.66 | .523 | |||||||
| thalamic k3 | 8.25 | .56 | 2.00 | .068 | |||||||
| cortical k3 | −21.73 | −.77 | −2.51 | .027 | |||||||
To address the caveat that our behavioral data were not collected on the same date as PET data, we conducted an additional regression analysis including the number of days between the PET session and the behavioral testing session as an additional control variable, entered in the first step with age. There was no relationship between this variable and the distractor effect (b*=.35, t=1.52, p=.152), and more importantly, the relationship between cortical k3 and the distractor effect was not substantially affected (from b*=−.77, p = .027 without this additional control variable to b*=−.75, p = .024 when it is included). We also tested a model in which the dopaminergic medication dosage (LEDD) was entered in the first step as an additional control variable along with age. LEDD was not a significant predictor (b*=−.25, t=−1.11, p=.286); nor did its inclusion in the model substantially change the contributions of thalamic k3 (from b*=−.77, p = .027 to b*=−.78, p = .032).
Cholinergic functions in subregions of cortex and distractibility
As an exploratory analysis, we evaluated which cortical region provides the best AChE k3 predictor for distractibility. Mean k3 values were extracted from 33 cortical regions separately for left and right hemispheres (thus 66 cortical subregions) and the thalamus. The cortical regions were segmented using Freesurfer (https://surfer.nmr.mgh.harvard.edu/) and the subcortical structures (e.g., caudate nucleus, putamen) were manually segmented using Interactive Data Language image analysis software (Research systems, Inc., Boulder, CO; See Supplemental Methods for more details).
Next, bivariate correlation analysis was used to assess the association between k3 and distractor effect in each of these regions. These analyses should be interpreted with caution due to low sample size and lack of correction for multiple comparisons, but provide a preliminary indication that may be useful for constraining hypotheses in future studies with larger sample sizes and more power. Greater distractor vulnerability was associated with low levels of k3 in the left middle frontal gyrus (MFG), broad parietal regions, and ventral temporal regions (Figure 4). No region showed a positive correlation.
Figure 4. Regional-specificity of the correlations between the cholinergic integrity and distractor effect.
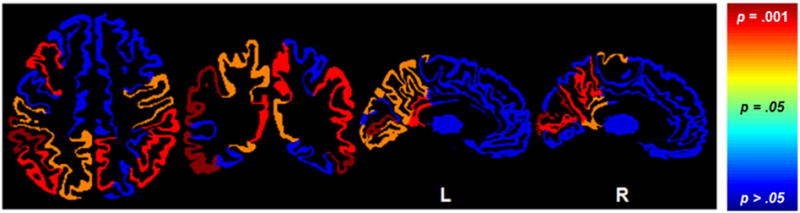
Greater distractor vulnerability was associated with lower cholinergic integrity in the left middle frontal gyrus, bilateral posterior cingulate, parietal and temporal regions. p = .01 = r value of −.61; p = .05 = r value of −.48.
Discussion
The results of the present study suggest that cortical cholinergic innervation plays an important and specific role in the ability to resist distraction from external sources, rather than simply reflecting global cognitive decline and dementia. Compared to HC, PD patients had only marginal impairments on the measure of initial performance thought to reflect attentional focus and precision in duration judgments, and the two groups had similar declines in sustained attention over time. Likewise, when looking within the PD group, cortical cholinergic denervation was significantly correlated with the distractor effect but not with the ability to focus attention or sustain performance over time. Regression analyses provided further support by showing that the cortical-cholinergic/distraction relation not only remained, but became even stronger after controlling for age, caudate dopaminergic, and thalamic cholinergic measures1. Finally, regional analyses provided preliminary support for the idea that left fronto-parietal cholinergic integrity may be of particular importance.
The conclusion that cholinergic innervation of the cortex plays a critical role in the ability to resist external sources of distraction takes on additional interpretative power when the present results are considered in combination with other findings. In particular, Berry et al. (2014b) tested participants with a genetic polymorphism thought to reduce cholinergic function ((the Ile89Val variant of the choline transporter (CHT) gene SLC5A7 (rs1013940)) in the same paradigm used here, and likewise found a specific vulnerability to distraction, with no deficits in initial performance or in the ability to sustain performance over time. Other studies have also found that genetic variation in the CHT is associated with variation in reactivity to biologically or emotionally (rather than perceptually) salient stimuli (Gorka et al., 2015; Neumann et al., 2006). However, these genetic polymorphisms presumably affect cholinergic efficiency throughout the entire brain. To the best of our knowledge, the present study represents the first direct evidence in humans for specifically cortical-cholinergic involvement in the ability to resist external distractors.
There are a number of indirectly supportive findings from animal models and other patient studies. Of particular interest, Kucinski et al. (2017) found that in a “dual-lesion” (striatal dopaminergic and basal forebrain-cortical cholinergic) rodent model of PD, pro-cholinergic drug treatment specifically reduced freezing and falls associated with an irrelevant visual distractor – a “doorframe” meant to model the conditions that often lead to freezing in patients with PD (Cowie et al., 2012).
In addition to looking at individual differences within PD samples, as we did here, comparisons across different patient populations provide evidence that cortical and thalamic cholinergic innervation make relatively independent contributions to cognition. For example, in Alzheimer’s disease, degeneration is largely along the basal forebraincortical cholinergic pathway, with very little thalamic cholinergic denervation (less than 1%) relative to controls. In contrast, progressive supranuclear palsy primarily affects the thalamic cholinergic pathway and largely spares cortical cholinergic innervation (Gilman et al., 2010). Alzheimer’s patients accordingly have primarily cognitive deficits, including an increased vulnerability to distraction, whereas progressive supranuclear palsy patients primarily have difficulties with motor function and susceptibility to falls correlated with thalamic volume and function (e.g., Baddeley et al., 2001; Gilman et al., 2010; Zwergal et al., 2011; see discussion by Sidiropoulos and LeWitt, 2011).
Our previous study (Kim et al., 2017) also supports the hypothesis of distinct roles for thalamic versus cortical cholinergic pathways. In that study, the target stimulus was a sudden-onset visual signal (small dot presented briefly in the center of the screen), and thus had a great deal of “bottom-up” perceptual salience (Posner, 1978), especially in comparison to the target in the current study, for which detection depended on accurate mental representations of duration. Using the same regression models as we did here, we found that thalamic, not cortical, cholinergic integrity predicted successful detection of that “bottom-up” cue, suggesting that the thalamic cholinergic pathway plays a critical role in processing signal salience.
In the present study, thalamic cholinergic integrity did not show any relation to performance unless cortical cholinergic integrity was also included in the model – in which case there was a trend (β = .56, p = .07) for better thalamic cholinergic integrity to predict greater vulnerability to distraction, whereas better cortical cholinergic integrity was associated with less vulnerability to the distractor. In regression terms, this suggests a suppressor effect – that in this case might also be interpreted as inhibition at a cognitive level. That is, it suggests the interesting possibility that thalamic cholinergic integrity governs the degree to which the video distractor (which presumably has high perceptual salience) attracts attention along bottom-up attentional pathways, whereas cortical cholinergic integrity impacts the degree to which top-down attention acts to suppress the salient distractor and/or quickly return attention to the target task. As noted by one of our reviewers, the regions where cortical cholinergic integrity was associated with the ability to maintain performance despite the distractor generally show strong overlap with those supporting visual attention and working memory (e.g., Corbetta & Shulman, 2002; Hopfinger & Mangun, 2000.
This brings up an important limitation of our study: A larger sample size would be required to test potential interactions between the predictors in the model. Besides the possible interactions between the cortical and thalamic components noted above, cortical cholinergic decline occurs in the context of dopaminergic decline and treatment. This is of particular interest as some studies suggest that patients off dopaminergic medication may have reduced vulnerability to external distraction, whereas patients on medication may have increased distractor vulnerability compared to both the “off” state and healthy controls (Cools et al., 2010; Uitvlugt et al., 2016). However, as noted above, including LEDD in the model did not substantially affect estimates of the cortical cholinergic contribution to distractor resistance. As a further test, we also examined a “kitchen sink” model (not included in the Results section due to collinearity) in which MoCA, BDI, AES, PAC score, and disease duration were entered in the first step, along with age. Although collinearity between the predictor variables becomes an issue for this model, cortical cholinergic integrity remained the only PET measure significantly related to the distractor effect.
Likewise, the regional cerebral analyses should also be considered exploratory and hypothesis-generating, rather than hypothesis-testing. We include them here to provide potential targets for future research, and because they somewhat contradict our initial expectations, and we felt it was important to acknowledge this. The primary difference between the present findings and our earlier empirical and theoretical work is the laterality of the effects. In the present study, the regions with the strongest correlations between cholinergic denervation and vulnerability to the distractor effect included the middle frontal gyrus and parietal regions, both on the left. Our previous studies have likewise consistently found that fronto-parietal regions, especially middle/inferior frontal gyrus and superior parietal lobule, are associated with meeting increased demands for top-down control (e.g., Berry et al., 2015; Berry et al., in press; Demeter et al., 2011). However, in those previous studies, as well as in parallel animal studies, both fMRI activation and cholinergic outcome measures have been right-lateralized.
Of course, one plausible explanation for this discrepancy is that the current study is under-powered for these analyses. A large-scale study would be required to rule this out. However, a more interesting possibility is that these fronto-parietal regions perform related operations, but at different levels of complexity or on different types of representations. Specifically, we have proposed that the right-lateralized effects, especially in right middle/inferior frontal gyrus, reflect “attentional effort”, or the motivated reinforcement of rule and goal representations at the overall task or goal level, especially in the face of challenges to attention (e.g., Berry et al., in press; Lustig & Sarter, 2016; St, Peters et al., 2011; see also Jimura et al., 2010; Raizada & Poldrack, 2007 for related concepts). In contrast, left-lateralized effects may reflect more detailed, granular-level operations involved in the analysis of stimulus or response options to determine if they are consistent with those broader goals. This would also be consistent with the well-known role that left frontal regions play in language processing, perhaps especially important since the distractor videos used here (clips from newscasts, sports shows, sitcoms, documentaries, etc.) had a strong verbal/semantic component.
To explore this possibility, we conducted a series of reverse inference term-based meta-analyses using the Neurosynth database (Yarkoni et al., 2011). First, we searched for the term “distraction”, in keeping with our original hypotheses for this experiment. This revealed a right prefrontal cluster (40, 16, 38; z = 5.99, 66 studies) near the one we had previously identified as associated with the term “task difficulty” (46, 6, 32) and those identified in our studies using the signal-detection task; see Berry et al. (in press) for details. However most of the studies included in this effect used relatively simple visual distractors (motion, color, or shape singletons in visual search tasks, or distractor stimuli easily distinguishable from targets (e.g., faces vs houses)). No similar left-lateralized cluster was observed.
In contrast, a search for the term “interference”, which is usually defined as competition between different stimuli, memory representations, or response options, reveals nearby clusters that are bilateral, but stronger on the left (−40, 6, 30; z = 6.73, 271 studies). The majority of these were working-memory studies, with most using letters, words, or other semantic content (e.g., meaningful pictures) as stimuli, and distractors that were quite similar to targets (in many cases, having been targets on previous trials, and serving as lures on the current trial) and thus requiring a relatively detailed analysis to determine which items should be accepted as targets, and which to reject. In addition, grey matter density in left parietal regions has been associated with resistance to real-world distraction, and disruption of this region using transcranial magnetic stimulation increases susceptibility to distraction (Kanai, Dong, Bahrami, & Rees, 2011). Returning to the rodent studies, although most have focused on right-lateralized effects and successful target detection, some findings suggest that left-hemispheric cortical cholinergic function supports the ability to reject irrelevant inputs, and that lesioning the left fronto-parietal cholinergic system leads to an increase in false alarms (e.g., Martinez & Sarter, 2004). Again, this proposed lateralization of function should be considered an interesting hypothesis to guide future research, not a strong conclusion based on the current findings.
What the present study, in combination with Kim et al. (2017), can provide is evidence linking thalamic and cortical cholinergic pathways to specific cognitive functions, beyond the degree usually possible in pharmacologic or genetic studies. It thus begins to build a bridge between the more sophisticated, precise work on such pathways that can be done in animal models and the likewise more sophisticated and precise tests of cognitive function that can be performed in humans. We hope that both the data and the hypotheses we have presented here help guide future research to further advance our basic understanding of brain-behavior relationships, and to ultimately improve interventions for patients with many disorders who have difficulty regulating the processing of relevant environmental stimuli while avoiding distraction.
Supplementary Material
Acknowledgments
The authors thank all patients and volunteers for their time commitment. We are also very grateful to Christine Minderovic for her work as patient coordinator. This work was supported by the Department of Veterans Affairs [grant number I01 RX000317]; the Michael J. Fox Foundation; and the NIH [grant numbers P01 NS015655 and RO1 NS070856 with additional support from P50 NS091856]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests.
When the same regression model was applied to initial performance and declines in performance over time (slope), none of the included variables was a significant predictor over and above the others. The only relationship in these analysis to approach significance was between initial performance and the caudate dopamine measure (b* = −.52, t = −1.77, p = .10), perhaps consistent with the established role of dopamine in temporal judgments (e.g., Meck, 1996).
References
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression Possible implications for functional neuropathology. The British Journal of Psychiatry. 2001;178(3):200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124(8):1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37(3):379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, Role LW. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron. 2016;91(6):1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berry AS, Blakely RD, Sarter M, Lustig C. Cholinergic capacity mediates prefrontal engagement during challenges to attention: evidence from imaging genetics. Neuroimage. 2015;108:386–395. doi: 10.1016/j.neuroimage.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Li X, Lin Z, Lustig C. Shared and distinct factors driving attention and temporal processing across modalities. Acta psychologica. 2014a;147:42–50. doi: 10.1016/j.actpsy.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M, Lustig C. Disposed to Distraction: Genetic Variation in the Cholinergic System Influences Distractibility But Not Time-on-Task Effects. Journal of Cognitive Neuroscience. 2014b;26(9):1981–1991. doi: 10.1162/jocn_a_00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Sarter M, Lustig C. Distinct Frontoparietal Networks Underlying Attentional Effort and Cognitive Control. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_01112. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behavioural Brain Research. 2011;221(2):564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Frey KA. Imaging of cholinergic and monoaminergic neurochemical changes in neurodegenerative disorders. Molecular Imaging and Biology. 2007;9(4):243–257. doi: 10.1007/s11307-007-0083-6. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis CA, Davis JG, Moore RY, DeKosky ST. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. Journal of Neurology. 2006;253(2):242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Müller MLTM, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. Journal of Cerebral Blood Flow & Metabolism. 2012;32(8):1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133(1):225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-directed attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Hariz M, Day BL. Doorway-provoked freezing of gait in Parkinson’s disease. Movement disorders. 2012;27(4):492–499. doi: 10.1002/mds.23990. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Depression and Parkinson’s disease: A review. The American Journal of Psychiatry. 1992;149(4):443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. NeuroImage. 2011;54(2):1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Danzé F, Pillon B, Cusimano G, Lhermitte F, Agid Y. Cholinergic-dependent cognitive deficits in Parkinson’s disease. Annals of Neurology. 1987;22(1):26–30. doi: 10.1002/ana.410220108. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B, Lhermitte F, Agid Y. Cholinergic deficiency and frontal dysfunction in Parkinson’s disease. Annals of Neurology. 1990;28(2):117–121. doi: 10.1002/ana.410280202. [DOI] [PubMed] [Google Scholar]
- Dunois B, Ruberg M, Javoy-Agid F, Ploska A, Agid Y. A subcortico-cortical cholinergic system is affected in Parkinson’s disease. Brain research. 1983;288(1):213–218. doi: 10.1016/0006-8993(83)90096-3. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Field A, Miles J, Field Z. Discovering Statistics: Using R. Sage Publication Ltd, Washington, DC. Fritz, C. O., Morris, P. E., & Richler, J. J. Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74(18):1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn M. The Apathy Evaluation Scale. The Center for Outcome Measurement in Brain Injury; 2005. [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, Lewitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, Lapelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disordoder. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Grondin S. Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Attention, Perception, & Psychophysics. 2010;72(3):561–582. doi: 10.3758/APP.72.3.561. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huba GJ, Singer JL, Aneshensel CS, Antrobus JS. Short imaginal processes inventory. Research Psychologists Press; Port Hurson, MI: 1982. [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Current Opinion in Neurobiology. 2004;14(2):225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Dong MY, Bahrami B, Rees G. Distractibility in daily life is reflected in the structure and function of human parietal cortex. The Journal of Neuroscience. 2011;31(18):6620–6626. doi: 10.1523/JNEUROSCI.5864-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neuro-Degenerative Diseases. 2013;11(2):79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Müller MLTM, Bohnen NI, Sarter M, Lustig C. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson’s disease patients with defined cholinergic losses. NeuroImage. 2017;149:295–304. doi: 10.1016/j.neuroimage.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Jong IE, Sarter M. Reducing falls in Parkinson’s disease: interactions between donepezil and the 5-HT6 receptor antagonist idalopirdine on falls in a rat model of impaired cognitive control of complex movements. European Journal of Neuroscience. 2017;45:217–231. doi: 10.1111/ejn.13354. [DOI] [PubMed] [Google Scholar]
- Kilbourn M, Snyder SE, Sherman PS, Kuhl DE. In vivo studies of acetylcholinesterase activity using a labeled substrate, N-[WIMethylpiperdin-4-yl Propionate. Synapse. 1996;22:123–131. doi: 10.1002/(SICI)1098-2396(199602)22:2<123::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA, Kilbourn MR. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology. 1999;52(4):691–691. doi: 10.1212/wnl.52.4.691. [DOI] [PubMed] [Google Scholar]
- Kuhl D, Koeppe R, Snyder S, Minoshima S, Frey K, Kilbourn M. Mapping acetylcholinesterase activity in human brain using PET and N [11C]Methylpiperidinyl propionate (PMP) J Nucl Med. 1996a;37(Suppl):21P. [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, Ficaro EP, Wieland DM, Koeppe RA, Frey KA, Foster NL. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996b;40(3):399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of Brain States. Neuron. 2012;76(1):209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Sarter M. Attention and the cholinergic system: relevance to schizophrenia. Curr Top Behav Neurosci. 2016:1–36. doi: 10.1007/7854_2015_5009. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Lateralized attentional functions of cortical cholinergic inputs. Behavioral neuroscience. 2004;118(5):984. doi: 10.1037/0735-7044.118.5.984. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3(3):227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D 2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacology Biochemistry and Behavior. 1986;25(6):1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal–striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Müller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 2013;136(11):3282–3289. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MLTM, Bohnen NI. Cholinergic Dysfunction in Parkinson’s Disease. Current Neurology and Neuroscience Reports. 2013;13(9):1–9. doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. The Journal of Neuroscience. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychological Methods. 2003;8(4):434. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89(1–3):125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends in Neurosciences. 1999;22(6):273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, Perry RH. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1985;48(5):413–421. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Cusimano G, Bonnet AM, Lhermitte F, Agid Y. Does cognitive impairment in Parkinson’s disease result from non-dopaminergic lesions? Journal of Neurology, Neurosurgery & Psychiatry. 1989;52(2):201–206. doi: 10.1136/jnnp.52.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Reviews Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raizada RD, Poldrack RA. Challenge-driven attention: Interacting frontal and brainstem systems. Frontiers in human neuroscience. 2007;1 doi: 10.3389/neuro.09.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer TH. On dopaminergic modulation of temporal information processing. Biological Psychology. 1993;36(3):209–222. doi: 10.1016/0301-0511(93)90018-4. [DOI] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian Journal of Psychology. 2002;43(3):239–251. doi: 10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Blakely RD, Cherian AK. Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. Journal of Physiology-Paris. 2016;110(1):10–18. doi: 10.1016/j.jphysparis.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulos C, LeWitt PA. Localizing imbalance in progressive supranuclear palsy Is the thalamus the “fall guy”? Neurology. 2011;77(2):92–93. doi: 10.1212/WNL.0b013e318223c7b1. [DOI] [PubMed] [Google Scholar]
- Singer JL, Antrobus JS. Imaginal processes inventory 1970 [Google Scholar]
- St Peters MS, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced Control of Attention by Stimulating Mesolimbic–Corticopetal Cholinergic Circuitry. The Journal of Neuroscience. 2011;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in parkinson’s disease: A community-based study. Archives of Neurology. 1996;53(2):175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Uitvlugt MG, Pleskac TJ, Ravizza SM. The nature of working memory gating in Parkinson’s disease: A multi-domain signal detection examination. Cognitive, Affective, & Behavioral Neuroscience. 2016;16(2):289–301. doi: 10.3758/s13415-015-0389-9. [DOI] [PubMed] [Google Scholar]
- Varela C. Thalamic neuromodulation and its implications for executive networks. Front Neural Circuit. 2014;8:69. doi: 10.3389/fncir.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Mahler HR, Moore WJ. The half-life of acetylcholinestrerase in mature rat brain. J Neurochem. 1974;22(6):941–943. doi: 10.1111/j.1471-4159.1974.tb04319.x. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cerebral Cortex. 2015a;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J. Organization of the basal forebrain cholinergic projection system: specific or diffuse? In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 2015b. pp. 491–507. [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Current Directions in Psychological Science. 1997:12–16. [Google Scholar]
- Zwergal A, La Fougere C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, Linn J, Krafczyk S, Dieterich M, Brindt T, Strupp M, Bartenstein P, Jahn K. Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology. 2011;77(2):101–109. doi: 10.1212/WNL.0b013e318223c79d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


