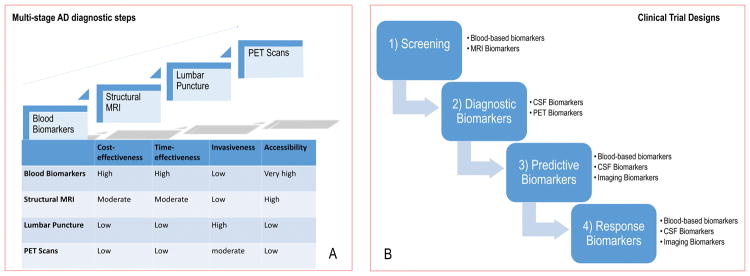
Figure 8. Evolving spectrum of biomarkers and modalities.
A. The ideal biomarker should be minimally-invasive, unexpansive, practical, rapid and reliable with low level of expertise required. Therefore, in the clinical-setting, biomarkers should be assessed in a multi-stage diagnostic workout carried-out along four steps (blood biomarkers, structural MRI, lumbar puncture, PET scans) according to the overall balance among the following factors: cost-effectiveness, time-effectiveness, invasiveness and accessibility. B. Biomarkers represent one strategy to tailor therapy. The idealistic markers for ND would enable their implementation in screening, diagnosis, progression of the disease, and monitoring of the response to therapy. Therefore, in clinical trials, biomarkers can be used for several purposes:
1) to identify people eligible for the trial, i.e. those considered at high risk for ND (screening biomarkers),
2) to guide clinical diagnosis (diagnostic markers),
3) to optimize treatment decisions, providing information on the likelihood of response to a given drug (predictive biomarkers),
4) to detect and quantify the response rate to treatment (response markers).
Abbreviations: MRI, magnetic resonance imaging; PET, Positron Emission Tomography; ND, neurodegenerative diseases.