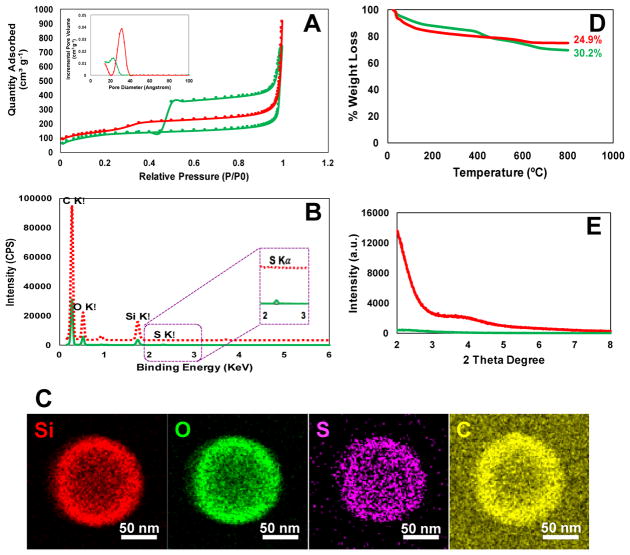
Fig. 2.
(A) Nitrogen adsorption desorption isotherms of the fabricated HMSiO2 NPs with/without hysteresis loop which show type IV isotherms according to IUPAC classification. Inset is the pore size distribution plots for each nanoparticle shown in angstrom; (B) STEM spectra for both HMSiO2 NPs. GSH-sensitive HMSiO2 NPs exhibit a small peak around 2.3 KeV which is ascribed to the presence of sulfur; (C) STEM images of GSH-sensitive HMSiO2 NPs demonstrate homogenous distribution of sulfur in the outer shell; (D) TGA graphs show c.a. 5.3% more weight loss in GSH-sensitive HMSiO2 NPs in comparison with TEOS HMSiO2 NPs attributed to the presence of organosilane matter in disulfide-based particles; (E) XRD plots of the synthesized HMSiO2 NPs confirm disordered pore structure in GSH-sensitive HMSiO2 NPs and well-ordered pore structure in TEOS HMSiO2 NPs due to the observation of two broad Bragg peaks. (Note: green lines and red lines/dash represent GSH-sensitive HMSiO2 NPs and TEOS HMSiO2 NPs, respectively).