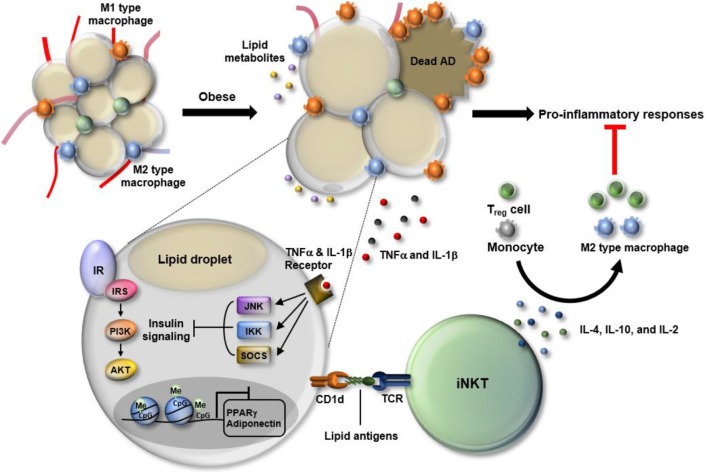
Figure 1.
Dynamic changes in white adipose tissue (WAT) immunity in obesity. In the progression of obesity, WAT faces multiple stresses, including hypoxia, oxidative stress, and epigenetic malfunction. Particularly, adipocytes become enlarged in the process of absorbing excess nutrients, which is accompanied by adipocyte death and leakage of lipid metabolites. Also, obesity-induced DNA hypermethylation in adipocytes leads to the suppression of genes involved in adipocyte function including peroxisome proliferator-activated receptor gamma (PPARγ) and adiponectin. In response to such changes, WAT immunity skews toward pro-inflammatory state. Among cells residing in WAT, M1-type macrophages secrete a variety of cytokines, such as TNF-α and interleukin (IL)-1β that activate JNK, IKK, and SOCS, leading to suppression of insulin signaling in WAT. Adipose invariant natural killer T (iNKT) cells have anti-inflammatory roles as a part of defense mechanism to resolve pro-inflammatory responses. Adipose iNKT cells are mainly activated by lipid antigens loaded onto adipocyte CD1d and secrete Th2-type cytokines, such as IL-4, IL10, and IL-2. Those cytokines drive the polarization of monocytes toward M2 type macrophages and activate regulatory T cells, contributing to an alleviation of the pro-inflammatory responses in WAT.