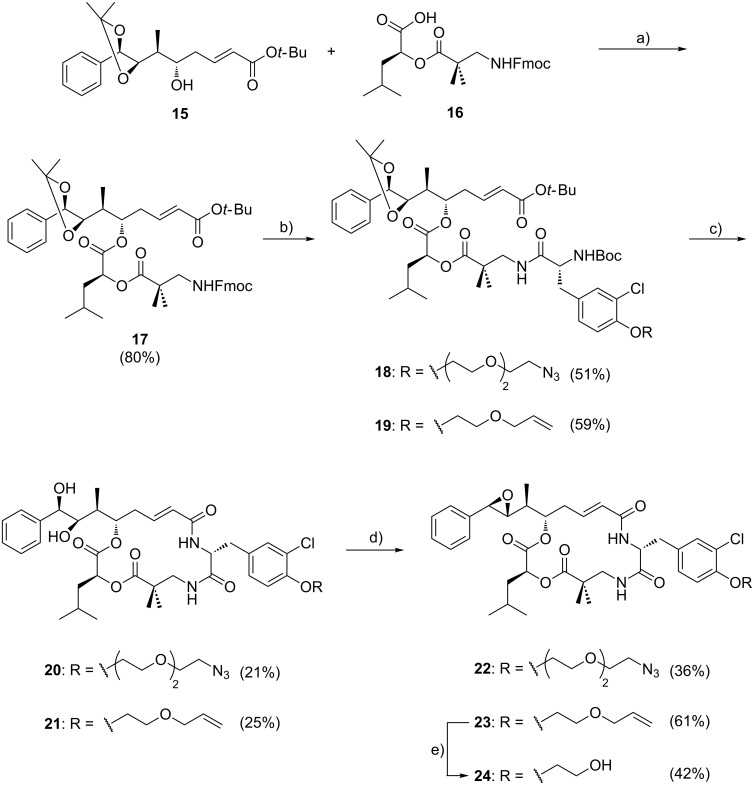
Scheme 2.
Synthesis of cryptophycin analogues 22, 23 and 24. Reagents and conditions: (a) 4-DMAP, 2,4,6-trichlorobenzoyl chloride, Et3N, THF, 0 °C, 3 h; (b) 1) piperidine, DMF, rt, 2 h; 2) 13 or 14, HOAt, EDC·HCl, Et3N, CH2Cl2, 0 °C → rt, overnight; (c) 1) TFA/CH2Cl2/H2O, rt, 2 h; 2) HATU, HOAt, DIPEA, DMF, rt, slow addition + 2 h; (d) 1) (CH3O)3CH, PPTS, CH2Cl2, rt, 2 h; 2) AcBr, CH2Cl2, rt, 4 h; 3) K2CO3, DME/ethylene glycol (2:1 v/v), rt, 5 min; (e) Pd(PPh3)4, phenylsilane, CH2Cl2, rt, 7 h.