Table 4.
Thermal cycloaddition of sydnones with terminal alkynes.
 | |||||||
| entry | R1 | R2 | R3 | conditions | ratio 1,3:1,4 |
yield [%]a | ref. |
| 1 | Ph | Me | n-Hex | xylene, 140 °C, 30 h | n.d. | 78 | [1] |
| 2 | Ph | H | n-Hex | toluene, 111 °C, 52 h xylene, 160 °C, 24 h |
n.d. 90:10 |
72 65 |
[2] [91] |
| 3 | Ph | H | Ph | chlorobenzene, 120 °C, 20 h xylene, 140 °C, 16 h o-DCB, μ-wave, 200 °C, 2 h o-DCB, 140 °C, 24 h |
n.d. >95:5 91:9 91:9 |
79/<2 35 66 62 |
[1–2] [82] [84] [92] |
| 4 | Ph | Me | Ph | 140 °C, 12 h 142 °C, 7 h |
~80:20 ~89:11 |
64/15 73/9 |
[1] [2] |
| 5 | Bn | H | Ph | xylene, 135–140 °C, 20 h | 100:0 | 69–74 | [1–2] |
| 6 | Ph | H | COOMe | xylene, 100 °C, 48 h sc-CO2, 60–160 °C, 7.6 MPa |
76:24 85:15–76:24 |
70/22 – |
[1–2] [93] |
| 7 | Ph | Me | COOMe | 140 °C, 4 h xylene, reflux, 1 h |
n.d. 65:35 |
61/10 55/29 |
[1] [2] |
| 8 | Ph | H | CH(OPr)2 | xylene, 135–140 °C, 3 h | n.d. | 28/58 | [2] |
| 9 | Ph | Me | CH(OPr)2 | xylene, 135–140 °C, 15 h | n.d. | 77 | [1] |
| 10 | Bn | H | CH(OPr)2 | xylene, 135–140 °C, 15 h | n.d | 78 | [2] |
| 11 | Ph | H | CH2OH | reflux, 24 h | 100:0 | 66–72 | [1–2] |
| 12 | Ph | H | CN | chlorobenzene, 110 °C, 24 h | 100:0 | 50 | [66] |
| 13 | NMe2 | H | Ph | tetraline, reflux, 5 h | n.d. | 60/– | [67] |
| 14 | NMe2 | H | 4-Cl-Ph | tetraline, reflux, 5 h | n.d. | 23/– | [67] |
| 15 | NMe2 | H | 4-Me-Ph | tetraline, reflux, 5 h | n.d. | 32/– | [67] |
| 16 | NMe2 | H | n-Hex | tetraline, reflux, 5 h | n.d. | 50/– | [67] |
| 17 | O(CH2CH2)2N | H | Ph | tetraline, reflux, 5 h | n.d. | 22/– | [67] |
| 18 | (CH2)5N | H | 4-Cl-Ph | tetraline, reflux, 5 h | n.d. | 24/1 | [67] |
| 19 | NMe2 | Me | Ph | tetraline, reflux, 5 h | n.d. | 81/10 | [67] |
| 20 | NMe2 | Me | 4-Cl-Ph | tetraline, reflux, 5 h | n.d. | 30/4 | [67] |
| 21 | NMe2 | O(CH2CH2)2NCH2 | Ph | tetraline, reflux, 5 h | n.d. | 34/2 | [67] |
| 22 | NMe2 | O(CH2CH2)2NCH2 | 4-Cl-Ph | tetraline, reflux, 5 h | n.d. | 12/2 | [67] |
| 23 | Ph | MeS | COOMe | toluene, 95–105 °C, 12.5 h | 46:54 | 39/50 | [8] |
| 24 | Ph | PhS | COOMe | xylene, 140 °C, 35 h | 53:47 | 95 | [8] |
| 25 | Ph | MeSO | COOMe | mesitylene, 135–140 °C, 19 h | 81:19 | 65/15 | [8] |
| 26 | Ph | MeCO | COOMe | mesitylene, 155–160 °C, 90 h | 60:40 | 46/37 | [8] |
| 27 | Ph | Ph | COOMe | xylene, 110–115 °C, 12 h o-DCB, reflux, 48 h |
50:50 50:50 |
40/44 97 |
[8] [80] |
| 28 | 4-NO2-Ph | Ph | COOMe | toluene, 95–105 °C, 16 h | 56:44 | 51/37 | [8] |
| 29 | 2,4-diNO2-Ph | Ph | COOMe | toluene, 100–105 °C, 18.5 h | 61:39 | 55/36 | [8] |
| 30 | 4-NO2-Ph | H | COOMe | toluene, 95–105 °C, 4 h | 86:14 | 99 | [8] |
| 31 | Ph | H | PhSO2 | toluene, 100 °C, 24 h | 25:75 | 56 | [68] |
| 32 | CH2CH2CH2 | Ph | xylene | ≈75:25 | 51/18 | [69] | |
| 33 | Ph | I | COOMe | xylene, reflux, 24 h | 58:42 | n.d. | [20] |
| 35 | 2-Et-Ph | I | COOMe | xylene, reflux, 24 h | 56:44 | n.d. | [20] |
| 36 | Me | H | COOMe | toluene, reflux, 12 h | 100:0 | 75 | [70] |
| 37 | CH2CH2CH2CH2 | COOMe | xylene, reflux, 10 h xylene, reflux, 16 h xylene, reflux, 6 h |
67:33 n.d. n.d. |
60 65/26 56/– |
[71] [77] [83] |
|
| 38 | CH2CH2CH2CH2 | COOEt | xylene, reflux, 10 h | 75:25 | 75 | [71] | |
| 39 | CH2CH2CH2CH2 | COOn-Bu | xylene, reflux, 10 h | 63:37 | 72 | [71] | |
| 40 | CH2CH2CH2CH2 | COOBn | xylene, reflux, 10 h | 69:31 | 59 | [71] | |
| 41 | CH2CH2CH2CH2 | COO(1-PhEt) | xylene, reflux, 10 h | 66:34 | 60 | [71] | |
| 42 | 4-Br-2-Et-Ph | I | COOEt | xylene, reflux, 24 h | – | – | [24] |
| 43 |  |
COOMe | o-xylene, reflux, 15 h | n.d. | 68/12 | [72,77] | |
| 44 | CH2SCH2 | COOMe |
o-xylene, reflux, 19 h xylene, reflux, 4 h |
n.d. | 49/22 53/21 |
[72–73] [36] |
|
| 45 |  |
COOMe | o-xylene, reflux, 16 h | n.d. | 64/24 | [72,77] | |
| 46 |  |
COOMe | o-xylene, reflux, 16 h | n.d. | 32/32 | [72] | |
| 47 |  |
COOMe |
o-xylene, reflux, 16 h o-xylene, reflux, 15 h |
n.d. | 59/34 68/12 |
[72] [73] |
|
| 48 |  |
COOMe | o-xylene, reflux, 21 h | 40:60 | 80 | [72] | |
| 49 | CH2CH2CH2 | COOMe | xylene, reflux, 8 h 1,2-diethoxyethane, 120–125°C, 8 h |
n.d. ≈87:13 |
40/35 47 |
[13] [74] |
|
| 50 | 4-EtO-Ph | H | COOEt | chlorobenzene, reflux, 48 h | 76:24 | 90 | [75] |
| 51 | 4-EtO-Ph | I | COOEt | chlorobenzene, reflux, 48 h | 56:44 | 81 | [75] |
| 52 | 4-EtO-Ph | CN | COOEt | chlorobenzene, reflux, 48 h | 58:42 | 80 | [75–76] |
| 53 | 4-EtO-Ph | CH2OH | COOEt | chlorobenzene, reflux, 48 h | 63:37 | 71 | [75] |
| 54 | 4-EtO-Ph | PhS | COOEt | chlorobenzene, reflux, 48 h | 52:48 | 71 | [75] |
| 55 | 4-EtO-Ph | CN | COOBn | chlorobenzene, reflux, 48 h | 57:43 | 76 | [75–76] |
| 56 | 4-EtO-Ph | CN | COOt-Bu | chlorobenzene, reflux, 48 h | 78:22 | 79 | [75–76] |
| 57 | 4-EtO-Ph | CN | COOCHPh2 | chlorobenzene, reflux, 48 h | 100:0 | 85 | [75–76] |
| 56a | Ph | CN | COOCHPh2 | chlorobenzene, reflux, 48 h | 100:0 | 80 | [75–76] |
| 57a |  |
COOMe | o-xylene, reflux, 21 h | n.d. | 87 | [77] | |
| 58 | 2,3-diMe-Ph | H | COOMe | xylene, reflux, 12 h | 75:25 | n.d. | [28] |
| 59 | Ph | H | Me3Si | toluene, reflux | n.d. | 95/– | [78] |
| 60 | Ph | H | Me2PhSi | toluene, reflux | n.d. | 80/– | [78] |
| 61 | Ph | H | t-BuPh2Si | toluene, reflux | n.d. | 15/– | [78] |
| 62 | Ph | H | BPin | mesitylene, reflux, 16 h | 88:12 | 47/7 | [79,81] |
| 63 | Ph | Ph | 4-(Me2N)-Ph | o-DCB, reflux, 48 h | n.d. | 65/– | [80] |
| 64 | 4-NO2-Ph | Me | BPin | o-DCB, reflux, 24 h | 89:11 | 79 | [81] |
| 65 | 4-NO2-Ph | iPr | BPin | o-DCB, reflux, 24 h | >98:2 | 75 | [81] |
| 66 | CH2CH2CH2CH2 | BPin | xylene, reflux, 24 h | 90:10 | 78 | [81] | |
| 67 | 4-NO2-Ph | H | Ph | xylene, 140 °C, 8 h | 95:5 | 60 | [82] |
| 68 | 4-NO2-Ph | I | Ph | xylene, 140 °C, 8 h | 91:9 | 84 | [82] |
| 69 | Ph | I | Ph | xylene, 140 °C, 16 h | >95:5 | 73 | [82] |
| 70 | 4-MeO-Ph | H | Ph | xylene, 140 °C, 24 h o-DCB, 140 °C, 24 h |
91:9 91:9 |
30 76 |
[82] [92] |
| 71 | 4-MeO-Ph | I | Ph | xylene, 140 °C, 24 h | 91:9 | 72 | [82] |
| 72 | 4-NO2-Ph | H | Me3Si | xylene, 140 °C, 8 h | 89:11 | 75b | [82] |
| 73 | 4-NO2-Ph | I | Me3Si | xylene, 140 °C, 8 h | 95:5 | 99b | [82] |
| 74 | 4-NO2-Ph | H | n-Bu | xylene, 140 °C, 8 h | 91:9 | 47b | [82] |
| 75 | 4-NO2-Ph | I | n-Bu | xylene, 140 °C, 8 h | 91:9 | 82b | [82] |
| 76 | 4-NO2-Ph | I | Bn | xylene, 140 °C, 8 h | 91:9 | 64b | [82] |
| 77 | 4-NO2-Ph | I | cyclo-Pr | xylene, 140 °C, 8 h | 94:6 | 77b | [82] |
| 78 | 4-NO2-Ph | I | CH2OBn | xylene, 140 °C, 8 h | >95:5 | 62b | [82] |
| 79 | 4-NO2-Ph | I | C(OH)Ph2 | xylene, 140 °C, 8 h | >95:5 | 70b | [82] |
| 80 | 4-NO2-Ph | I | 4-MeO2C-Ph | xylene, 140 °C, 8 h | >95:5 | 65b | [82] |
| 81 | 3-Py | H | Ph | o-DCB, μ-w, 200 °C, 2 h | 89:11 | 84 | [84] |
| 82 | Ph | H | 2-Py 2-PyH+ TsO– |
o-DCB, μ-w, 200 °C, 2 h ethylene glycol, reflux, 16 h |
60:40 91:9 |
85 14 |
[84] |
| 83 | 3-Py | H | 2-Py | o-DCB, μ-w, 200 °C, 2 h | 67:33 | 80 | [84] |
| 84 | Ph | H |  |
o-DCB, μ-w, 200 °C, 2 h | 60:40 | 86 | [84] |
| 85 | 3-Py | H |  |
o-DCB, μ-w, 200 °C, 2 h | 60:40 | 84 | [84] |
| 86 | 4-NO2-Ph | Me | 2-Py | o-DCB, reflux, 20 h | 80:20 | 87 | [84] |
| 87 | 4-NO2-Ph | iPr | 2-Py | o-DCB, reflux, 20 h | 86:24 | 78 | [84] |
| 88 | 4-NO2-Ph | iPr | 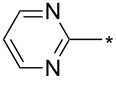 |
o-DCB, reflux, 20 h | n.d. | 66/19 | [84] |
| 89 | 3-Py | H | BPin | mesitylene, reflux, 16 h | 89:11 | 84 | [84] |
| 90 | CH2CH2CH2 | 4-F-Ph | mesitylene, 155–160 °C, 16 h | n.d. | 27/– | [85] | |
| 91 | Ph | CF3 | Ph | o-DCB, 180 °C, 24 h | 94:6 | 87 | [32–33] |
| 92 | Ph | CF3 | cyclo-Pr | o-DCB, 180 °C, 24 h | >98:2 | 88 | [32–33] |
| 93 | Ph | CF3 | Me3Si | o-DCB, 180 °C, 24 h | >98:2 | 75 | [32–33] |
| 94 | Ph | CF3 | 2-Py | o-DCB, 180 °C, 24 h | 95:5 | 84 | [32–33] |
| 95 | Ph | CF3 | BnOCH2 | o-DCB, 180 °C, 24 h | 96:4 | 84 | [32–33] |
| 96 | Ph | CF3 | 2-F-4-Cl-5-Me-Ph | o-DCB, 180 °C, 24 h | n.d. | 86/– | [32] |
| 97 | Ph | CF3 | Bu | o-DCB, 180 °C, 24 h | >98:2 | 78 | [33] |
| 98 | Ph | CF3 |  |
o-DCB, 180 °C, 24 h | >98:2 | 89 | [33] |
| 99 | Ph | CF3 | (CH2)3Cl | o-DCB, 180 °C, 24 h | 98:2 | 70 | [33] |
| 100 | 4-MeO-Ph | CF3 | Ph | o-DCB, 180 °C, 24 h | >98:2 | 85 | [33] |
| 101 | 4-MeO-Ph | CF3 | Bu | o-DCB, 180 °C, 24 h | >98:2 | 71 | [33] |
| 102 | 4-MeO-Ph | CF3 | cyclo-Pr | o-DCB, 180 °C, 24 h | >98:2 | 75 | [33] |
| 103 | 4-NO2-Ph | CF3 | Ph | o-DCB, 180 °C, 24 h | >98:2 | 85 | [33] |
| 104 | 4-NO2-Ph | CF3 | (CH2)3Cl | o-DCB, 180 °C, 24 h | >98:2 | 68 | [33] |
| 105 | Me | CF3 | Ph | o-DCB, 180 °C, 24 h | 98:2 | 95 | [33] |
| 106 | Me | CF3 |  |
o-DCB, 180 °C, 24 h | 98:2 | 82 | [33] |
| 107 | Me | CF3 | BnOCH2 | o-DCB, 180 °C, 24 h | >98:2 | 89 | [33] |
| 108 | Me | CF3 | COOEt | o-DCB, 180 °C, 24 h | 93:7 | 94 | [33] |
| 109 | Ph | CF3 | BPin | o-DCB, 140 °C, 72 h | 93:7 | 69 | [32–33] |
| 110 | Me | CF3 | BPin | o-DCB, 140 °C, 72 h | 96:4 | 44 | [33] |
| 111 | Bn | CF3 | Ph (2 equiv) Ph (2 equiv) Ph (2 equiv) Ph (10 equiv) |
o-DCB, 180 °C, 24 h o-DCB, 140 °C, 24 h o-DCB, 140 °C, 48 h o-DCB, 180 °C, 24 h |
64:36 96:4 88:12 88:12 |
61 34 66 64 |
[33] |
| 112 | Bn | CF3 | Bu | o-DCB, 180 °C, 24 h | 72:28 | 48 | [33] |
| 113 | Ph | CH2OH | Ph | o-DCB, 180 °C, 24 h | n.d. | 72/– | [33] |
| 114 | Ph |  |
COOEt | o-DCB, 180 °C, 30 min, μ-wave | 88:12 | 66 | [34] |
| 115 | Ph |  |
Ph | xylene, 140 °C, 6 h, μ-wave | 98:2 | 51 | [34] |
| 116 | Ph |  |
Me3Si | xylene, 140 °C, 3.5 h, μ-wave | 98:2 | 17 | [34] |
| 117 | 4-MeO-Ph |  |
COOEt | o-DCB, 180 °C, 1 h, μ-wave | 83:17 | 44 | [34] |
| 118 | Bn |  |
COOEt | o-DCB, 180 °C, 30 min, μ-wave | 67:33 | 21 | [34] |
| 119 | Ph | H |  |
toluene, reflux, 12 h | 100:0 | 33 | [86] |
| 120 | 4-Me-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 35 | [86] |
| 121 | 4-I-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 40 | [86] |
| 122 | 4-Cl-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 43 | [86] |
| 123 | 4-F-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 38 | [86] |
| 124 | 4-MeO-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 33 | [86] |
| 125 | 3,4-OCH2O-Ph | H |  |
toluene, reflux, 12 h | 100:0 | 30 | [86] |
| 126 | Ph | H |  |
toluene, reflux, 12 h | ≈34:67 | 18/36 | [86] |
| 127 | 4-Me-Ph | H |  |
toluene, reflux, 12 h | ≈40:60 | 28/42 | [86] |
| 128 | 4-I-Ph | H |  |
toluene, reflux, 12 h | ≈80:20 | 20/5 | [86] |
| 129 | 4-Cl-Ph | H |  |
toluene, reflux, 12 h | ≈83:17 | 35/4 | [86] |
| 130 | 4-F-Ph | H |  |
toluene, reflux, 12 h | ≈67:33 | 20/10 | [86] |
| 131 | 4-MeO-Ph | H |  |
toluene, reflux, 12 h | ≈20:80 | 10/40 | [86] |
| 132 | 3,4-OCH2O-Ph | H |  |
toluene, reflux, 12 h | ≈67:33 | 20/10 | [86] |
| 133 |  |
CF3 | o-xylene, −78–270 °C, 12 h | n.d. | 61/10 | [87] | |
| 134 | Ph | H | 3,5-di-HC≡C-Ph | N-methylpyrrolidone, 185 °C, 48 h | n.d. | (32) | [88] |
| 135 | 3,4,5-tri-MeO-Ph | 3-BnO-4-MeO-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 74 | [89] |
| 136 | 3-BnO-4-MeO-Ph | 3,4,5-tri-MeO-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 79 | [89] |
| 137 | Me | H | 3,4,5-tri-MeO-Ph | xylene, 160 °C, 24 h | >98:2 | 88 | [89] |
| 138 | Bn | H | 3,4,5-tri-MeO-Ph | xylene, 160 °C, 24 h | 90:10 | 65 | [89] |
| 139 | Me | H | 3-TBSO-4-MeO-Ph | xylene, 160 °C, 24 h | 90:10 | 65 | [89] |
| 140 | Bn | H | 3-TBSO-4-MeO-Ph | xylene, 160 °C, 24 h | 90:10 | 58 | [89] |
| 141 | 4-MeO-Ph | 4-MeO-Ph | COOEt | o-DCB, 140–180 °C, 16 h | 50:50 | <60 | [90] |
| 142 | 4-MeO-Ph | 4-MeO-Ph | 3-CN-4-Cl-Ph-CO | o-DCB, 140 °C, 16 h | 50:50 | 95 | [90] |
| 143 | 4-MeO-Ph | 4-MeO-Ph | CH2OH | xylene, 160 °C, 24 h | 93:7 | 97 | [90] |
| 144 | 4-MeO-Ph | 3,4,5-tri-MeO-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 100 | [91] |
| 145 | 3,4,5-tri-MeO-Ph | 3-NH2-4-MeO-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 91 | [91] |
| 146 | Ph | CON(Me)OMe | Me3Si | xylene, 160 °C, 24 h | 95:5 | 89 | [91] |
| 147 | Bn | 3-CF3-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 77 | [91] |
| 148 | 3,4,5-tri-MeO-Ph | 3-OH-4-MeO-Ph | Me3Si | xylene, 160 °C, 24 h | 95:5 | 82 | [91] |
| 149 | Ph |  |
Me3Si | xylene, 160 °C, 24 h | 95:5 | 87 | [91] |
| 150 | 4-F-Ph | H | Ph | xylene, 160 °C, 24 h | 90:10 | 100 | [91] |
| 151 | 4-MeO-Ph | H | cyclo-Pr | xylene, 160 °C, 24 h | 90:10 | 91 | [91] |
| 152 | Ph |  |
cyclo-Pr | xylene, 160 °C, 24 h | 95:5 | 91 | [91] |
| 153 | Ph | 4-Me-Ph | CH2OH | xylene, 160 °C, 24 h | 95:5 | 63 | [91] |
| 154 | Ph | CON(Me)OMe | cyclo-Pr | xylene, 160 °C, 24 h | 95:5 | 84 | [91] |
| 155 | Ph | 2-Py | Ph | xylene, 160 °C, 24 h | 95:5 | 98 | [91] |
| 156 | Ph | H | COOEt | o-DCB, 140 °C, 16 h | 67:33 | 59 | [92] |
| 156 | 4-MeO-Ph | H | COOEt | o-DCB, 140 °C, 24 h | 67:33 | 57 | [92] |
aIsolated yield of single or both regioisomers. bIn a sealed tube. n.d. – not determined.
