Table 5.
Thermal cycloaddition of sydnones with internal non-symmetrical alkynes.
 | ||||||||
| entry | R1 | R2 | R3 | R4 | conditions | ratio a:b |
yield [%]a | ref. |
| 1 | Ph | H | Me | Ph | xylene, 135–140 °C, 20 h | n.d. | 83/– | [1–2] |
| 2 | Ph | H | COOEt | Ph | toluene, 95 °C, 84 h | n.d. | 82–83/– | [1–2] |
| 3 | 4-Cl-Ph | H | COOEt | Ph | xylene, reflux, 3 h | n.d. | 92/– | [2] |
| 4 | 4-MeO-Ph | H | COOEt | Ph | xylene, reflux, 3 h | n.d. | 83/– | [2] |
| 5 | 4-Me-Ph | H | COOEt | Ph | xylene, reflux, 3 h | n.d. | 98/– | [2] |
| 6 | Bn | H | COOEt | Ph | xylene, reflux, 16 h | n.d. | 46/– | [2] |
| 7 | Ph | Ph | COOEt | Ph | p-cymene, 160 °C, 16 h | n.d. | 87/– | [2] |
| 8 | Ph | Me | COOEt | Ph | xylene, 110 °C, 8 h | 100:0 | 82 | [1–2] |
| 9 | Ph | H | COMe | Ph | chlorobenzene, 130 °C, 12 h | 100:0 | 100 | [1–2] |
| 10 | Ph | H | COPh | Ph | xylene, 135–140 °C, 16 h | 100:0 | 82 | [1–2] |
| 11 | Me | H | COPh | Ph | o-DCB, reflux, 144 h | 69:31 | 99 | [16] |
| 12 | Ph | H | CN | Cl | chlorobenzene, 110 °C, 10 h | n.d. | 15/20 | [66] |
| 13 | Ph | H | SO2Ph | Me | toluene, 100 °C, 24 h | 100:0 | 58 | [68] |
| 14 | Ph | H | SO2Ph | Ph | toluene, 100 °C, 24 h | 100:0 | 73 | [68] |
| 15 | Ph | H | COOMe | CH(OMe)2 | toluene, reflux, 60 h | 21:79 | 84 | [99] |
| 16 | Bn | H | COOMe | CH(OMe)2 | toluene, reflux, 72 h | 19:81 | 80 | [99] |
| 17 | Bn | H | COOMe | CHO | toluene, reflux, 18 h | 72:28 | 90 | [99] |
| 18 | Ph | H | COOMe | CHO | toluene, reflux, 18 h | 66:34 | 93 | [99] |
| 19 | Bn | H | COOMe | CH2OH | toluene, reflux, 72 h | 50:50 | 75 | [99] |
| 20 | Ph | H | COOMe | CH2OH | toluene, reflux, 48 h | 60:40 | 79 | [99] |
| 21 | Ph | H | CF3 | 4-MeO-Ph | xylene, 120 °C, 48–72 h | 93:7 | 56 | [100] |
| 22 | Ph | H | CF3 | 4-NO2-Ph | xylene, 120 °C, 48–72 h | 93:7 | 93 | [100] |
| 23 | Ph | H | CF3 | 4-MeS-Ph | xylene, 120 °C, 48–72 h | 93:7 | 90 | [100] |
| 24 | Ph | H | CF3 | 2-Cl-Ph | xylene, 120 °C, 48–72 h | 94:6 | 92 | [100] |
| 25 | Ph | H | CF3 | 4-MeSO2-Ph | xylene, 120 °C, 48–72 h | 92:8 | 86 | [100] |
| 26 | Ph | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 93:7 | 75 | [100] |
| 27 | 4-Cl-Ph | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 93:7 | 90 | [100] |
| 28 | 4-MeO-Ph | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 93:7 | 84 | [100] |
| 29 | Bn | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 91:9 | 65 | [100] |
| 30 | t-Bu | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 93:7 | 58 | [100] |
| 31 | Me | H | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 92:8 | 92 | [100] |
| 32 | Ph | Me | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 84:16 | 75 | [100] |
| 33 | Ph | 4-Cl-Ph | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 60:40 | 57 | [100] |
| 34 | Ph | Br | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 71:29 | 73 | [100] |
| 35 | Ph | MeS | CF3 | 4-Cl-Ph | xylene, 120 °C, 48–72 h | 43:57 | 62 | [100] |
| 36 | t-Bu | H | COOEt | Et | xylene, reflux, 72 h | n.d. | 38/8 | [101] |
| 37 | Ph | H | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 74 | [102] |
| 38 | Ph | H | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 80 | [102] |
| 39 | Ph | H | 4-Cl-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 72 | [102] |
| 40 | 4-MeO-Ph | H | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 73 | [102] |
| 41 | 4-MeO-Ph | H | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 74 | [102] |
| 42 | 4-MeO-Ph | H | 4-Cl-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 73 | [102] |
| 43 | 4-Me-Ph | H | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 79 | [102] |
| 44 | 4-Me-Ph | H | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3-4 h | 100:0 | 83 | [102] |
| 45 | 4-Me-Ph | H | 4-Cl-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | 100:0 | 75 | [102] |
| 46 | Me | H | COOEt | CF3 | xylene, 100 °C, 4 h | n.d. | 18/25 | [103] |
| 47 | Ph | H | SnBu3 | SiMe3 | toluene, reflux | 100:0 | 80 | [78] |
| 48 | Ph | H | SiMe2Ph | SiMe3 | toluene, reflux | n.d. | 63/34 | [78] |
| 49 | Ph | H | COMe | SiMe3 | toluene, reflux | n.d. | 81/16 | [78] |
| 50 | Ph | H | BPin | Ph | xylene, reflux, 4 h | 98:2 | 58 | [79,81] |
| 51 | Ph | H | BPin | Bu | xylene, reflux, 4 h | 71:29 | 64 | [79,81] |
| 52 | Ph | H | BPin | Me3Si | xylene, reflux, 4 h | 67:33 | 76 | [79,81] |
| 53 | 4-MeO-Ph | H | BPin | Ph | xylene, reflux, 4 h | 98:2 | 58 | [79] |
| 54 | 4-NO2-Ph | H | BPin | Ph | xylene, reflux, 4 h | 98:2 | 70 | [79] |
| 55 | 4-MeO-Ph | H | BPin | Bu | xylene, reflux, 4 h | 83:17 | 55 | [79] |
| 56 | 4-NO2-Ph | H | BPin | Bu | xylene, reflux, 4 h | 83:17 | 62 | [79] |
| 57 | 4-MeO-Ph | H | BPin | Me3Si | xylene, reflux, 4 h | 67:33 | 61 | [79] |
| 58 | 4-NO2-Ph | H | BPin | Me3Si | xylene, reflux, 4 h | 60:40 | 83 | [79] |
| 59 | 3-Py | H | BPin | Ph | xylene, reflux, 16 h | >98:2 | 60 | [84] |
| 60 | 3-Py | H | BPin | Me3Si | xylene, reflux, 16 h | 57:43 | 70 | [81,84] |
| 61 | 3-Py | H | BPin | n-Bu | mesitylene, reflux, 16 h | 71:29 | 56 | [84] |
| 62 | 4-Me-Ph | CHO | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 79/– | [104] |
| 63 | 4-Me-Ph | CHO | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 74/– | [104] |
| 64 | 4-Me-Ph | Br | 4-MeO-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 62/– | [104] |
| 65 | 4-MeO-Ph | Br | 4-MeO-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 69/– | [104] |
| 66 | 4-Me-Ph | Br | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 73/– | [104] |
| 67 | 4-Me-Ph | Br | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 66/– | [104] |
| 68 | 4-MeO-Ph | Br | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 63/– | [104] |
| 69 | Ph | Br | 4-Me-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 72/– | [104] |
| 70 | 4-MeO-Ph | MeCO | PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 74/– | [104] |
| 71 | 4-MeO-Ph | MeCO | 4-MeO-PhCO | 5-NO2-furan-2-yl | xylene, reflux, 3–4 h | n.d. | 73/– | [104] |
| 72 | Ph | H | 4-Me-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 71/– | [105] |
| 73 | Ph | H | 4-MeO-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 73/– | [105] |
| 74 | 4-Me-Ph | H | PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 75/– | [105] |
| 75 | 4-Me-Ph | H | 4-Me-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 73/– | [105] |
| 76 | Ph | H | 4-Cl-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 72/– | [105] |
| 77 | 4-Me-Ph | H | 4-Cl-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 77/– | [105] |
| 78 | 4-MeO-Ph | H | 4-Cl-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 78/– | [105] |
| 79 | Ph | H | PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 80/– | [105] |
| 80 | 4-MeO-Ph | H | PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 75/– | [105] |
| 81 | 4-MeO-Ph | H | 4-Me-PhCO | 5-NO2-thiophen-2-yl | xylene, reflux, 3–4 h | n.d. | 76/– | [105] |
| 82 | CH2CH2CH2 | p-Tos | Bu | anisol, reflux, 0.5 h | n.d. | 90/– | [106] | |
| 83 | CH2CH2CH2 | p-Tos | Ph | anisol, reflux, 0.5 h | n.d. | 89/– | [106] | |
| 84 | Ph | 4-Me-Ph | BPin | Me3Si | o-DCB, reflux, 48 h | 100:0 | 48 | [80] |
| 85 | Ph | 4-NO2-Ph | BPin | Me3Si | o-DCB, reflux, 48 h | 100:0 | 70 | [80] |
| 86 | Me | H | BPin | Ph | mesitylene, reflux, 48 h | >98:2 | 53 | [81] |
| 87 | Bn | H | BPin | Ph | xylene, reflux | >98:2 | 62 | [81] |
| 88 | Ph | Ph | BPin | Ph | o-DCB, reflux, 48 h | >98:2 | 59 | [81] |
| 89 | Ph | Ph | BPin | Me3Si | o-DCB, reflux, 48 h | >98:2 | 73 | [81] |
| 90 | Me | Ph | BPin | Me3Si | o-DCB, reflux, 48 h | >98:2 | 68 | [81] |
| 91 | Ph | Me | BPin | Ph | o-DCB, reflux, 48 h | >98:2 | 53 | [81] |
| 92 | Ph | iPr | BPin | Ph | o-DCB, reflux, 48 h | >98:2 | 38 | [81] |
| 93 | Ph | Me | BPin | Me3Si | o-DCB, reflux, 48 h | >98:2 | 56 | [81] |
| 94 | Ph | iPr | BPin | Me3Si | o-DCB, reflux, 48 h | >98:2 | 43 | [81] |
| 95 | 4-NO2-Ph | Me | BPin | Ph | o-DCB, reflux, 18 h | >98:2 | 67 | [81] |
| 96 | 4-NO2-Ph | iPr | BPin | Ph | o-DCB, reflux, 24 h | >98:2 | 45 | [81] |
| 97 | 4-NO2-Ph | Me | BPin | Me3Si | o-DCB, reflux, 18 h | >98:2 | 80 | [81] |
| 98 | 4-NO2-Ph | iPr | BPin | Me3Si | o-DCB, reflux, 24 h | >98:2 | 69 | [81] |
| 99 | CH2CH2CH2 | BPin | Me3Si | xylene, reflux, 24 h o-DCB, 180 °C, 24 h |
>98:2 100:0 |
21 66 |
[81] [107] |
|
| 100 | CH2CH2CH2CH2 | BPin | Me3Si | xylene, reflux, 24 h | >98:2 | 79 | [81] | |
| 101 | CH2CH2CH2CH2 | BPin | Ph | xylene, reflux, 24 h | >98:2 | 70 | [81] | |
| 102 | CH2CH2CH2 | BPin | Ph | o-DCB, 180 °C, 72 h | 50:50 | 51 | [107] | |
| 103 | CH2CH2CH2CH2 |  |
4-F-Ph | mesitylene, 165 °C, 18 h | n.d. | 46/– | [108] | |
| 104 | CH2CH2CH2CH2 |  |
4-F-Ph | mesitylene, 165 °C, 18 h | n.d. | 66/– | [108] | |
| 105 |  |
 |
4-F-Ph | mesitylene, 140 °C, 4 h | n.d. | 30/– | [108] | |
| 106 |  |
 |
4-F-Ph | mesitylene, 140 °C, 4 h | n.d. | 45/– | [108] | |
| 107 |  |
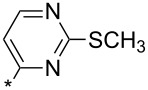 |
4-F-Ph | mesitylene, 160 °C, 24 h | n.d. | 12/– | [108] | |
| 108 | CH2SCH2 |  |
4-F-Ph | mesitylene, 160 °C, 48 h | n.d. | 13/– | [108] | |
| 109 | 4-Cl-Ph | H | Me |  |
mesitylene, 140 °C, 18 h | n.d. | 13/– | [109] |
| 110 | Ph | H |  |
Me3Si | o-DCB, μ-wave, 200 °C, 2 h | n.d. | 55/9 | [84] |
| 111 | Ph | I | COOEt | Br | toluene, reflux, 18 h | 76:24 | 52/16 | [110] |
| 112 | Me | I | COOEt | Br | xylene, reflux, overnight | 58:42 | 50/37 | [110] |
| 113 | Bn | I | COOEt | Br | xylene, reflux, overnight | 59:41 | 39/27 | [110] |
| 114 | 4-F-Ph | I | COOEt | Br | xylene, reflux, overnight | 75:25 | 48/16 | [110] |
| 115 | 4-MeO-Ph | I | COOEt | Br | xylene, reflux, overnight | 75:25 | 63/21 | [110] |
| 116 | 4-MeO-Ph | I | COOt-Bu | Br | xylene, reflux, overnight | 77:23 | 43/13 | [110] |
| 117 | 4-MeO-Ph | I | COOEt | I | xylene, reflux, overnight | 60:40 | 58/28 | [110] |
| 118 | 4-MeO-Ph | Br | COOEt | I | xylene, reflux, overnight | – | 0/0 | [110] |
| 119 | CH2CH2CH2CH2 | 4-Py | 4-F-Ph | mesitylene, 165 °C, 16 h | n.d. | 27/– | [85] | |
| 120 | Ph | H | COOEt | Br | toluene, reflux, 18 h | 46:54 | 41/48 | [111] |
| 121 | 4-Me-Ph | H | COOEt | Br | toluene, reflux, 18 h | 44:56 | 41/49 | [111] |
| 122 | 4-MeO-Ph | H | COOEt | Br | toluene, reflux, 18 h | 41:59 | 33/49 | [111] |
| 123 | 4-F-Ph | H | COOEt | Br | toluene, reflux, 18 h | 47:53 | 38/43 | [111] |
| 124 | Ph | CF3 | COOMe | Me | o-DCB, 180 °C, 24 h | 85:15 | 90 | [32] |
| 125 | Ph | CF3 | Ph | n-Bu | o-DCB, 180 °C, 24 h | 52:48 | 62 | [32] |
| 126 | Ph | CF3 | BPin | Me3Si | o-DCB, 140 °C, 48 h | 90:10 | 68 | [32] |
| 127 | Ph | CF3 | BPin | n-Bu | o-DCB, 140 °C, 72 h | >98:2 | 55 | [32] |
| 128 | Me | 3,4,5- triMeO-Ph |
BPin | Me3Si | xylene, 180 °C, 24 h | 83:17 | 92 | [89] |
| 129 | Bn | 3,4,5- triMeO-Ph |
BPin | Me3Si | xylene, 180 °C, 24 h | 90:10 | 66 | [89] |
| 130 | Me | 3-BnO- 4-MeO-Ph |
BPin | Me3Si | xylene, 180 °C, 24 h | 80:20 | 73 | [89] |
| 131 | Bn | 3-BnO- 4-MeO-Ph |
BPin | Me3Si | xylene, 180 °C, 24 h | 90:10 | 64 | [89] |
| 132 | Ph | 4-Me-Ph | BPin | Me3Si | xylene, 180 °C, 48 h | >98:2 | 74 | [91] |
| 133 | Ph | 2-Py | BPin | Me3Si | xylene, 180 °C, 48 h | >98:2 | 52 | [91] |
| 134 | Ph | 2-thienyl | BPin | Me3Si | xylene, 180 °C, 48 h | 88:12 | 64/6 | [91] |
| 135 | Me | 4-Me-Ph | BPin | Me3Si | xylene, 180 °C, 48 h | >98:2 | 55 | [91] |
| 136 | 4-EtO-Ph | 4-MeO-Ph | BPin | Me3Si | xylene, 180 °C, 48 h | 88:12 | 74 | [91] |
aIsolated overall yield or isolated yields of both regioisomers a/b. n.d. – not determined.
