Table 7.
Cu(I)-catalyzed cycloaddition of sydnones with terminal alkynes.
 | ||||||
| entry | R1 | R2 | R3 | ligand (L) | yield [%] | ref. |
| 1 | Ph | H | PhCH2CH2 | L1 L1 L2 |
96–98 85a 99 |
[3,119–120] [118] [3] |
| 2 | Ph | H | Ph | L1 | 80 | [3,120] |
| 3 | Ph | H | 4-MeOPh | L1 | 64 | [3,120] |
| 4 | Ph | H | 2-Py | L1 | 95 69a |
[3] [118] |
| 5 | Ph | H | thiophen-3-yl | L1 | 95 | [3,120] |
| 6 | Ph | H | 1-heptyl | L1 | 61 | [3,120] |
| 7 | Ph | H | PhCOOCH2 | L1 | 93 | [3,120] |
| 8 | Ph | H | (CH3)2C(OH) | L1 | 83 | [3,120] |
| 9 | Ph | H | COOEt | L1 | 95 51a |
[3,120] [118] |
| 10 | 4-COOH-Ph | H | PhCH2CH2 | L1 | 99 | [3,120] |
| 11 | 4-MeCO-Ph | H | PhCH2CH2 | L1 | 97 | [3] |
| 12 | 4-COOH-Ph | H | (CH3)2C(OH) | L1 | 93 | [3,120] |
| 13 | 4-COOH-Ph | H |  |
L1 | 99 | [3,120] |
| 14 | Ph | H |  |
L1 | 85 | [3] |
| 15 | Ph | H |  |
L1 | 85 | [3,120] |
| 16 | Ph | H |  |
L1 | 96 | [3,120] |
| 17 | Ph | H | 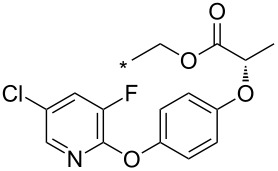 |
L1 | 62 55a |
[3,120] [118] |
| 18 | Ph | H |  |
L1 | 92 | [3,120] |
| 19 | Ph | H |  |
L1 | 84 | [3,120] |
| 20 |  |
H |  |
L1 | 99 | [3,120] |
| 21 | Ph | H | Bn–N–Ts | L2 | 64 | [116] |
| 22 | 4-F-Ph | H | Bn–N–Ts | L2 | 54 | [116] |
| 23 | 4-CF3-Ph | H | Bn–N–Ts | L2 | 57 | [116] |
| 24 | 4-MeO-Ph | H | Bn–N–Ts | L2 | 57 | [116] |
| 25 | 4-MeO-Ph | H | PhCH2CH2 | L1 | 69a | [118] |
| 26 | 4-Me-Ph | H | PhCH2CH2 | L1 | 72a | [118] |
| 27 | 4-I-Ph | H | PhCH2CH2 | L1 | 78a | [118] |
| 28 | 4-NO2-Ph | H | PhCH2CH2 | L1 | 69a | [118] |
| 29 | 4-CN-Ph | H | PhCH2CH2 | L1 | 92a | [118] |
| 30 | 4-COOH-Ph | H | PhCH2CH2 | L1 | 85a | [118] |
| 31 | 4-CF3-Ph | H | PhCH2CH2 | L1 | 80a | [118] |
| 32 | 3-I-Ph | H | PhCH2CH2 | L1 | 83a | [118] |
| 33 | naphthalen-1-yl | H | PhCH2CH2 | L1 | 69a | [118] |
| 34 | 2-COOMe-thiophen-3-yl | H | PhCH2CH2 | L1 | 50a | [118] |
| 35 | Ph | H | Ph | L1 | 84a | [118] |
| 36 | Ph | H | n-pentyl | L1 | 82a | [118] |
| 37 | Ph | H | CH2NHCOO-t-Bu | L1 | 85a | [118] |
| 38 | Ph | H |  |
L1 | 91a | [118] |
| 39 | Ph | Br | PhCH2CH2 | L1 L2 L3 L4 L5 L6 |
74b 67c 60d 75 74 13 |
[119–120] [119] [119] [119] [119] [119] |
| 40 | 4-Me-Ph | Br | PhCH2CH2 | L4 | 80 | [119] |
| 41 | 4-MeO-Ph | Br | PhCH2CH2 | L4 | 70 | [119–120] |
| 42 | 4-F-Ph | Br | PhCH2CH2 | L4 | 55 | [119] |
| 43 | 4-I-Ph | Br | PhCH2CH2 | L4 | 72 | [119] |
| 44 | Ph | Br | COOEt | L4 | 38 | [119] |
| 45 | Ph | Br | Ph | L4 | 63 | [119] |
| 46 | Ph | Br | 6-MeO-naphthalen-2-yl | L4 | 77 | [119] |
| 47 | Ph | Br | 4-MeO-Ph | L4 | 44 | [119] |
| 48 | Ph | Br | CH2NHCOO-t-Bu | L4 | 69 | [119] |
| 49 | Ph | Br | CH2OCOPh | L4 | 52 | [119] |
| 50 | Ph | Br | BrCH2CH2 | L1 L4 |
45a 63 |
[119] |
| 51 | quinolin-5-yl | Br | PhCH2CH2 | L4 | 33 | [119] |
| 52 | Ph | Br |  |
L4 | 52 | [119] |
| 53 | Ph | Br | 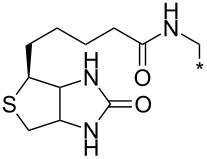 |
L4 | 65 | [119] |
| 54 | Ph | Me | PhCH2CH2 | L1 | 7 | [119] |
| 55 | Ph | Cl | PhCH2CH2 | L1 | 80e | [119] |
| 56 | Ph | CN | PhCH2CH2 | L1 | 10f | [119] |
aOne-pot protocol starting from corresponding N-phenyl glycine; bratio 1,4,5:1,3,5 is 83:17; cratio 1,4,5:1,3,5:1,4,5-debrominated product is 83:10:7; dratio 1,4,5:1,3,5:1,4,5-debrominated product is 97:0:3; eratio 1,4,5:1,3,5 is 96:4; fratio 1,4,5:1,3,5 is 50:50.
