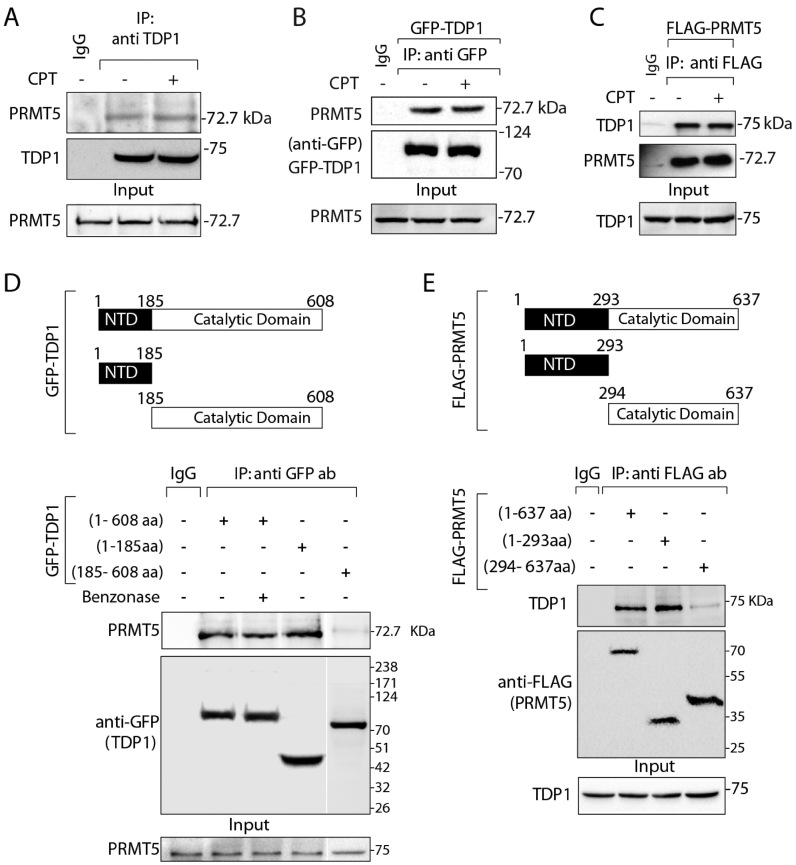
Figure 1.
PRMT5 physically interacts with TDP1. (A) Endogenous TDP1 from HCT116 cells treated with or without CPT (5 μM, 3 h) was immunoprecipitated using anti-TDP1 antibody and the immune complexes were blotted with anti-PRMT5 antibody. The same blot was stripped and reprobed with anti-TDP1 antibody. Aliquots (10%) of the input show the level of PRMT5 prior to immunoprecipitation. (B) HCT116 cells ectopically expressing GFP-TDP1 treated with or without CPT (5 μM, 3 h), and were immunoprecipitated using anti-GFP antibody. Immune complexes were blotted with anti-PRMT5 antibody. The same blot was stripped and reprobed with anti-GFP antibody to show the expression of the GFP-TDP1. Aliquots (10%) of the input show the level of PRMT5 prior to immunoprecipitation. (C) HCT116 cells ectopically expressing FLAG-PRMT5 were immunoprecipitated using anti-flag antibody and the immune complexes were blotted with anti-TDP1 antibody. The same blot was stripped and reprobed with anti-PRMT5 antibody. Aliquots (10%) of the input show the level of TDP1 prior to immunoprecipitation. (D) Schematic representation of GFP-tagged constructs showing full length (1-608 aa), truncated N-terminal domain (1–185 aa; N-terminal domain [NTD]), and truncated C-terminal domain (185–608 aa; catalytic domain) of human TDP1 are indicated. Ectopic GFP-TDP1 variants were expressed in HCT116 cells and immunoprecipitated using anti-GFP antibody and the immune complexes were probed with anti-PRMT5 antibody. To examine direct protein-protein interaction cell lysates were pretreated with benzonase prior to co-IP as indicated. Blots were subsequently stripped and probed with anti-GFP antibody to show the expression of the GFP-TDP1 variants. Aliquots (10%) of the input show the level of PRMT5 prior to immunoprecipitation. Migration of protein molecular weight markers (kDa) is indicated at right. (E) Schematic representation of flag-tagged constructs showing full length (1–637 aa), truncated N-terminal domain (1–293 aa) and truncated C-terminal domain (294–637 aa) of human PRMT5. Flag-tagged PRMT5 constructs were ectopically expressed in HCT116 cells and were co-immunoprecipitated with anti-flag antibody. The immune complexes were probed with anti-TDP1 antibodies. Blots were subsequently stripped and probed with anti-flag antibody to show the expression of FL and truncated constructs of Flag-PRMT5. Aliquots (10%) of the input show the level of TDP1 prior to immunoprecipitation. Migration of protein molecular weight markers (kDa) is indicated at right.