Abstract
A new series of tertiary amine derivatives of chlorochalcone (4a∼4l) were designed, synthesized and evaluated for the effect on acetylcholinesterase (AChE) and buthylcholinesterase (BuChE). The results indicated that all compounds revealed moderate or potent inhibitory activity against AChE, and some possessed high selectivity for AChE over BuChE. The structure–activity investigation showed that the substituted position of chlorine significantly influenced the activity and selectivity. The alteration of tertiary amine group also leads to obvious change in bioactivity. Among them, IC50 of compound 4l against AChE was 0.17 ± 0.06 µmol/L, and the selectivity was 667.2 fold for AChE over BuChE. Molecular docking and enzyme kinetic study on compound 4l suggested that it simultaneously binds to the catalytic active site (CAS) and peripheral anionic site (PAS) of AChE. Further study showed that the pyrazoline derivatives synthesized from chlorochalcones had weaker activity and lower selectivity in inhibiting AChE compared to that of chlorochalcone derivatives.
Keywords: Acetylcholinesterase inhibitors, Alzheimer’s disease, chlorochalcones, pyrazoline, structure–activity relationship
Introduction
Alzheimer’s disease (AD), as one of most common diseases in the elderly population, is a chronic and progressive neurodegenerative disorder1–3. Although the precise etiology of AD is not elucidated enough, acetylcholinesterase (AChE) inhibitors were confirmed to be as the primary drugs to slow down the progression of AD in the present4,5.
In recent, a lot of tertiary amine derivatives originated from natural products were synthesized and evaluated as AChE inhibitors6–9. The previous investigations in our laboratory suggested that chalcones with tertiary amine side chain, such as dimethylamine, diethylamine, dihydropyrrole or piperidine had more potent effects than other nitrogen-containing chalcones on inhibiting AChE10–12.
In order to explore the influence of substituent on inhibiting AChE in chalcone derivatives, halogen atoms were considered to introduce into the chalcone scaffold. Chlorine was selected to modify the chalcone derivatives, considering chlorine exist in a lot of drugs in clinic application, such as chlorphenamine, chlorpromazine, chloroquine, etc. In the investigations searching for AChE inhibitors, some chlorine-containing compounds revealed AChE inhibition properties13,14.
In this study, a series of chlorochalcones with tertiary amine side chain were synthesized and structure–activity relationship investigation was performed to explore the influence of chlorine in inhibiting AChE. In addition, four pyrazoline compounds were synthesized from chalcones and evaluated for the bioactivity to explore the influence of α,β-unsaturated ketone group. Compound 4l, which had the most potent inhibitory activity against AChE, was selected to perform the kinetic and molecular docking studies for exploring the binding mode or mechanism to AChE.
Materials and methods
Chemistry
All chemicals and reagents were of analytical reagent grade and used without further purification. The melting points were measured on a WRS-lA melting point detector1. H NMR spectra were recorded on a Bruker 400 MHz instrument in CDCl3 with TMS as the internal reference. Mass spectra were obtained on Finnigan LCQ advantage MAX by electrospray ionization (ESI-MS). Infrared spectrum (IR) was obtained on Shimadzu Infinity-1 infrared spectrometer. The purity of compounds was checked by Shimadzu LC-20A high-performance liquid chromatography. Elemental analysis was performed by elemental analyzer.
General method for synthesis of 4-hydroxy-chlorochalcones (3a–3c)
Compounds 3a–3c were synthesized according to the relative references15,16. 4-hydroxyacetophenone (1.36 g, 10 mmol) and ethanol (20 mL) were mixed and stirred in the flask for 10 min until the solid was dissolved. Then 30% NaOH (3 mL) was dropped into the mixture, followed by stirring for 30 min in ice bath. Then chlorine benzaldehyde (11 mmol) dissolving in ethanol (5 mL) was dropped into the mixture, and the mixture continued to stir for about 36 h at 25 °C monitoring by TLC until the reaction was completed. Then the pH of the mixture was adjusted to 2–3 by HCl (1 mol/L).The mixture was kept in ice bath for 2 h, followed by the appearance of precipitation. The precipitation was filtered and washed by distilled water, and then dried at 50 °C, refined by the recrystallization in anhydrous ethanol. In result, compounds 3a–3c were gained.
4'-Hydroxy-2-chlorochalcone (3a)
Light yellow solid was gained with yield of 80.7%. It was a known compound which was reported to have anticancer activity17.
4'-Hydroxy-3-chlorochalcone (3b)
Light yellow solid, yield: 73.5%; m.p: 1 2 0 ∼ 122 °C; 1H NMR (400 MHz, CDCl3, ppm): 6.96–6.98 (2H, d, J =8.0 Hz, 3′-H and 5′-H), 7.26–7.34 (2H, m, 4-H and 5-H), 7.54–7.81 (3H, m, α-H, 2-H and 6-H), 8.10–8.16 (3H, m, β-H, 2′-H and 6′-H), 11.10 (1H, s, OH). MS m/z (ESI): [M + H]+ 259. IR (KBr) ν/cm−1: 3426, 3016, 1667.2, 1617, 1583, 1446, 1340, 1288, 1215, 1172, 839 and 732. Anal. calcd for C15H11ClO2: C, 69.64; H, 4.29; O, 12.37; found C, 69.52; H, 4.27; N, 12.42%.
4'-Hydroxy -4-chlorochalcone (3c)
Light yellow solid was gained with yield of 85.6%. It is a known compound which was reported to inhibiting monoamine oxidases18.
General method for synthesis of 4-amino alkyl-chlorochalcones (4a–4l)
Compounds 4a–4l were synthesized according to the relative references19. 4’-hydroxy chlorochalcones (0.2620 g, 1 mmol) and potassium carbonate (0.4140 g, 3 mmol) were mixed in acetone (25 mL) in oil bath with continuous stirring at 56 °C for 30 min, then chloroethyldimethylamine hydrochloride, chloroethyldiethylamine hydrochloride, chloroethylpiperidine hydrochloride or chloroethylpyrrolidine hydrochloride (3 mmol) and sodium iodide (0.075 g, 0.5 mmol) were added into it. The mixture was refluxed overnight, filtered and concentrated. The concentrate was extracted with CH2Cl2 (3 × 30 mL), The CH2Cl2 phase was washed by saturated NaHCO3 (2 × 30 mL), saturated salt water (3 × 30 mL), dried with sodium sulfate anhydrous, followed by concentrating in vacuum and then the residue was purified by silica gel to gain the product with dichloromethane/methanol (80:1) as the eluent.
(E)-3–(2-chlorophenyl)-1–(4-(2-(dimethylamino)ethoxy)phenyl)prop-2-en-1-one (4a)
Light yellow solid, yield: 81.6%; m.p: 7 6 ∼ 77 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.09 (6H, s, 2 × NCH3), 2.92 (2H, t, J =12.0 Hz, OCH2CH2), 4.14 (2H, t, J =12.0 Hz, OCH2CH2), 6.98–7.00 (2H, m, 3′-H and 5′-H), 7.27–7.34 (2H, m, 3-H and 6-H), 7.43–7.77 (3H, m, α-H and 4-H and 5-H), 8.02–8.05 (2H, m, 2′-H and 6′-H), 8.15–8.19 (1H, d, J = 16.0 Hz, β-H). MS m/z (ESI): [M + H]+ 330. IR (KBr) ν/cm−1: 2806, 2769, 1661, 1611, 1576, 1458, 1261, 1227, 1176, 1020 and 730. Anal. calcd for C19H20ClNO2: C, 69.19; H, 6.11; N, 4.25; O, 9.70; found C, 68.28; H, 6.05; N, 4.21; O, 9.63.
(E)-3–(3-chlorophenyl)-1–(4-(2-(dimethylamino)ethoxy)phenyl)prop-2-en-1-one (4b)
Light white solid, yield: 82.6%; m.p: 6 2 ∼ 63 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.79 (6H, s, 2 × NCH3), 3.31 (2H, t, J = 12.0 Hz, OCH2CH2), 4.51 (2H, t, J =12.0 Hz, OCH2CH2), 6.96–6.98 (2H, d, J =8.0 Hz, 3′-H and 5′-H), 7.26–7.35 (2H, m, 4-H and 5-H), 7.54–7.81 (3H, m, α-H, 2-H and 6-H), 8.10–8.16 (3H, m, β-H, 2′-H and 6′-H), MS m/z (ESI): [M + H]+ 330. IR (KBr) ν/cm−1: 2805, 2774, 1678, 1626, 1576, 1458, 1246, 1227, 1175, 1020 and 722. Anal. calcd for C19H20ClNO2: C, 69.19; H, 6.11; N, 4.25; O, 9.70; found C, 69.32; H, 6.04; N, 4.19; O, 9.74.
(E)-3–(4-chlorophenyl)-1–(4-(2-(dimethylamino)ethoxy)phenyl)prop-2-en-1-one (4c)
A white solid, yield: 75.6%; m.p: 2 3 3 ∼ 234 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.37 (6H, s, 2 × NCH3), 2.79 (2H, t, J =12.0 Hz, OCH2CH2), 4.14 (2H, t, J =12.0 Hz, OCH2CH2), 7.00–7.02 (2H, m, 3′-H and 5′-H), 7.38–7.40 (2H, m, 2-H and 6-H), 7.51 (1H, d, J = 16.0 Hz, α-H), 7.55–7.59 (2H, m, 3-H and 5-H), 7.73–7.77 (1H, d, J = 16.0 Hz, β-H), 8.02–8.04 (2H, m, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 330. IR (KBr) ν/cm−1: 2805, 2774, 2359, 1658, 1595, 1558, 1456, 1246, 1227, 1175, 1020 and 731. Anal. calcd for C19H20ClNO2: C, 69.19; H, 6.11; N, 4.25; O, 9.70; found C, 68.98; H, 6.07; N, 4.28; O, 9.72.
(E)-3–(2-chlorophenyl)-1–(4-(2-(diethylamino)ethoxy)phenyl)prop-2-en-1-one (4d)
Yellow viscous liquid was gained with yield of 78.1%. It is a known compound with no reports about bioactivity20.
(E)-3–(3-chlorophenyl)-1–(4-(2-(diethylamino)ethoxy)phenyl)prop-2-en-1-one (4e)
Yellow viscous liquid, yield: 73.1%; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.12 (6H, t, J =12.0 Hz, 2 × NCH2CH3), 2.68–2.74 (4H, m, 2 × NCH2CH3), 2.96 (2H, t, J =12.0 Hz, OCH2CH2), 4.17 (2H, t, J =12.0 Hz, OCH2CH2), 6.96–6.98 (2H, d, J =8.0 Hz, 3′-H and 5′-H), 7.26–7.35 (2H, m, 4-H and 5-H), 7.54–7.81 (3H, m, α-H, 2-H and 6-H), 8.10–8.16 (3H, m, β-H, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 358. IR (KBr) ν/cm−1: 2970, 2810, 1661, 1508, 1337, 1246, 1173, 1024, 833 and 735. Anal. calcd for C21H24ClNO2: C, 70.48; H, 6.76; N, 3.91; O, 8.94; found: C, 70.63; H, 6.62; N, 3.85; O, 8.78.
(E)-3–(4-chlorophenyl)-1–(4-(2-(diethylamino)ethoxy)phenyl)prop-2-en-1-one (4f)
Yellow viscous liquid was gained with yield of 72.4%. It is a known compound with no reports about bioactivity21.
(E)-3–(2-chlorophenyl)-1–(4-(2-(piperidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4g)
Light white solid, yield: 83.7%; m.p: 5 5 ∼ 56 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.24–1.28 (2H, m, piperidine-H), 1.22–1.36 (4H, m, piperidine-H), 2.35–2.43 (4H, m, piperidine-H), 2.73(2H, t, J =12.0 Hz, OCH2CH2), 4.12 (2H, t, J =12.0 Hz, OCH2CH2), 6.98–7.00 (2H, m, 3′-H and 5′-H), 7.27–7.34 (2H, m, 3-H and 6-H), 7.43–7.77 (3H, m, α-H and 4-H and 5-H), 8.02–8.05 (2H, m, 2′-H and 6′-H), 8.15–8.19 (1H, d, J =16.0 Hz, β-H). MS m/z (ESI): [M + H]+ 370. IR (KBr) ν/cm−1: 3904, 3853, 3838, 3821, 3802, 3726, 3725, 3649, 2960, 2930, 2360, 1608, 1558, 1508, 1458 and 730. Anal. calcd for C22H24ClNO2: C, 71.44; H, 6.54; N, 3.79; O, 8.65 found C, 71.58; H, 6.41; N, 3.65; O, 8.72.
(E)-3–(3-chlorophenyl)-1–(4-(2-(piperidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4h)
Light yellow solid, yield: 85.7%; m.p: 7 5 ∼ 76 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.28–1.29 (2H, m, piperidine-H), 1.35–1.39 (4H, m, piperidine-H), 2.33–2.43 (4H, m, piperidine-H), 2.77 (2H, t, J =12.0 Hz, OCH2CH2), 4.15 (2H, t, J =12.0 Hz, OCH2CH2), 6.98–7.00 (2H, m, 3′-H and 5′-H), 7.27–7.34 (2H, m, 3-H and 6-H), 7.43–7.77 (3H, m, α-H and 4-H and 5-H), 8.02–8.05 (2H, m, 2′-H and 6′-H), 8.15–8.19 (1H, d, J =16.0 Hz, β-H). MS m/z (ESI): [M + H]+ 370. IR (KBr) ν/cm−1: 3903, 3853, 3838, 3802, 3736, 3649, 3566, 2960, 2934, 2359, 1659, 1611, 1578, 1236, 1175, 1026 and 730. Anal. calcd for C22H24ClNO2: C, 71.44; H, 6.54; N, 3.79; O, 8.65 found C, 71.52; H, 6.48; N, 3.71; O, 8.62.
(E)-3–(4-chlorophenyl)-1–(4-(2-(piperidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4i)
White solid was gained with the yield: 82.7%. It is a known compound with anticancer effect in a recent investigation22.
(E)-3–(2-chlorophenyl)-1–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4j)
Yellow solid was gained with the yield of 80.7%. It is a known compound with anticancer effect in a recent investigation22.
(E)-3–(3-chlorophenyl)-1–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4k)
White solid, yield: 80.7%; m.p: 9 7 ∼ 98 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.72–1.75 (4H, m, pyrrolidine-H), 2.56–2.58 (4H, m, pyrrolidine-H), 2.76 (2H, t, J =12.0 Hz, OCH2CH2), 4.01 (2H, t, J =12.0 Hz, OCH2CH2), 6.98–7.00 (2H, m, 3′-H and 5′-H), 7.27–7.34 (2H, m, 3-H and 6-H), 7.43–7.77 (3H, m, α-H and 4-H and 5-H), 8.02–8.05 (2H, m, 2′-H and 6′-H), 8.15–8.19 (1H, d, J =16.0 Hz, β-H). MS m/z (ESI): [M + H]+ 356. IR (KBr) ν/cm−1: 2956, 2781, 1657, 1604, 1593, 1510, 1479, 1339, 1269, 1211, 1175, 1020, 980 and 735. Anal. calcd for C21H22ClNO2: C, 70.88; H, 6.23; N, 3.94; O, 8.99; found C, 70.95; H, 6.27; N, 3.88; O, 8.91.
(E)-3–(4-chlorophenyl)-1–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)prop-2-en-1-one (4l)
Yellow solid was gained with the yield of 81.7%. It is a known compound with anticancer effect in a recent investigation22.
General procedure of synthesis of compounds 5a–5d
Compounds 4i, 4j, 4k or 4l (1 mmol), 80% hydrazine hydrate (0.2 mL, 4 mmol) and acetic acid (5 mL) were mixed and refluxed23. When the reaction was completed, the mixture was cooled to room temperature and 30 mL CH2Cl2 was added into it. The CH2Cl2 phase was washed by saturated K2CO3 solution, and dried by anhydrous sodium sulfate. After the solution was filtered and the solvent was evaporated under reduced pressure, the crude product was gained. The refined product was achieved by the silica column chromatography with the eluent (methanol: methylene chloride =1:20).
1–(5-(4-Chlorophenyl)-3–(4-(2-(piperidin-1-yl)ethoxy)phenyl)-4,5-dihydro-1H-pyrazol-1-yl) ethan-1-one (5a)
Yellow viscous liquid, yield: 70.6%; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.84–1.86 (6H, m, piperidine-H), 2.43 (3H, s, COCH3), 2.54–2.57 (4H, m, piperidine-H), 2.86 (2H, t, J =12.0 Hz, OCH2CH2), 3.07 (1H, dd, J =4.0 Hz, 16.0 Hz, CH2-Ha), 3.64 (1H, dd, J =12.0 Hz, 16.0 Hz, CH2-Hb), 4.20 (2H, t, J =12.0 Hz, OCH2CH2), 5.79–5.83 (1H, m, NCH), 6.92–6.95 (2H, m, 3′-H and 5′-H), 7.03–7.28 (4H, m, 1-H and 2-H and 4-H and 5-H), 7.65–7.67 (2H, m, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 426. IR (KBr) ν/cm−1: 3442, 3419, 3062, 3030, 2933, 2853, 2787, 1653, 1608, 1510, 1422, 1251, 1223, 1175, 1163, 1028, 988 and 814. Anal. calcd for C24H28ClN3O2: C, 67.67; H, 6.63; N, 9.87; O, 7.51; found C, 67.78; H, 6.52; N, 9.81; O, 7.46.
1–(5-(2-Chlorophenyl)-3–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)-4,5-dihydro-1H-pyrazol-1-yl) ethan-1-one (5b)
Yellow viscous liquid, yield:74.2%; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.84–1.87 (6H, m, piperidine-H), 2.69–2.73 (3H, s, COCH3), 2.96–2.99 (4H, m, piperidine-H), 3.09 (2H, t, J =12.0 Hz, OCH2CH2), 3.12 (1H, dd, J =4.0 Hz, 16.0 Hz, CH2-Ha), 3.76 (1H, dd, J =12.0 Hz, 16.0 Hz, CH2-Hb), 4.20 (2H, t, J =12.0 Hz, OCH2CH2), 5.76–5.80 (1H, m, NCH), 6.90–6.93 (2H, m, 3′-H and 5′-H), 7.13–7.35 (4H, m, 3-H and 4-H and 5-H and 6-H), 7.63–7.67 (2H, m, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 412. IR (KBr) ν/cm−1: 3446, 3421, 3063, 3032, 2935, 2858, 2779, 1642, 1590, 1512, 1339, 1243, 1219, 1173, 1020, 980 and 726. Anal. calcd for C23H26ClN3O2: C, 67.06; H, 6.36; N, 10.20; O, 7.77; found C, 66.91; H, 6.48; N, 10.09; O, 7.84.
1–(5-(3-Chlorophenyl)-3–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one (5c)
Yellow viscous liquid, yield:77.6%; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.84–1.87 (6H, m, piperidine-H), 2.69–2.73 (3H, s, COCH3), 2.96–2.99 (4H, m, piperidine-H), 3.09 (2H, t, J =12.0 Hz, OCH2CH2), 3.13 (1H, dd, J =4.0 Hz, 16.0 Hz, CH2-Ha), 3.76 (1H, dd, J =12.0 Hz, 16.0 Hz, CH2-Hb), 4.20 (2H, t, J =12.0 Hz, OCH2CH2), 5.76–5.80 (1H, m, NCH), 6.83–6.85 (2H, m, 3′-H and 5′-H), 7.04–7.29 (4H, m, 2-H and 4-H and 5-H and 6-H), 7.66–7.70 (2H, m, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 412. IR (KBr) ν/cm−1: 3443, 3417, 3062, 3032, 2936, 2881, 1645, 1583, 1510, 1479, 1339, 1269, 1243, 1211, 1175, 1020, 980 and 735. Anal. calcd for C23H26ClN3O2: C, 67.06; H, 6.36; N, 10.20; O, 7.77; found C, 66.89; H, 6.45; N, 10.34; O, 7.61.
1–(5-(4-Chlorophenyl)-3–(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)-4,5-dihydro-1H-pyrazol-1-yl) ethan-1-one (5d)
Yellow viscous liquid, yield: 73.2%; 1H NMR (400 MHz, CDCl3) δ (ppm): 1.84–1.87 (6H, m, piperidine-H), 2.69–2.73 (3H, s, COCH3), 2.96–2.99 (4H, m, piperidine-H), 3.09 (2H, t, J =12.0 Hz, OCH2CH2), 3.13 (1H, dd, J =4.0 Hz, 16.0 Hz, CH2-Ha), 3.76 (1H, dd, J =12.0 Hz, 16.0 Hz, CH2-Hb), 4.20 (2H, t, J =12.0 Hz, OCH2CH2), 5.76–5.80 (1H, m, NCH), 6.92–6.95 (2H, m, 3′-H and 5′-H), 7.03–7.28 (4H, m, 1-H and 2-H and 4-H and 5-H), 7.65–7.67 (2H, m, 2′-H and 6′-H). MS m/z (ESI): [M + H]+ 412. IR (KBr) ν/cm−1: 3442, 3419, 3060, 3031, 2930, 2883, 1640, 1585, 1510, 1307, 1263, 1230, 1221, 1175, 1032, 978 and 738. Anal. calcd for C23H26ClN3O2: C, 67.06; H, 6.36; N, 10.20; O, 7.77; found C, 67.24; H, 6.18; N, 10.12; O, 7.65.
Log p measurement
Log p, defined as the logarithm of octanol-water partition coefficient is an important parameter to evaluate the lipophilicify of compounds. It can be calculated by determining the concentration of compound in octanol phase and water phase until the partition equilibrium was completed. In present investigation, log p of compounds 4a∼4l was measured by the shake flask method with slight modification, and PBS (pH =7.4) was used as the water phase24. The mobile phase was methanol: 0.1% triethylamine (TEA)/80:20 (v/v), at a flow rate of 1.0 mL.min−1 through a C18 column (250 nm ×4.6 mm, 5 µm) at 32 °C with detect wavelength 318 nm. Experiments were conducted in triplicate and log p values were calculated.
AChE and BuChE inhibition assay
AChE/BuChE activity assays were conducted by Ellman method with light modification25. The brain and serum of Sprague–Dawley rat was used as the resource of AChE and BuChE, respectively. Each compound was dissolved in Tween 80 and diluted with water to various concentrations immediately before use. The reaction mixture containing 40 µL AChE/BuChE, 100 µL acetylthiocholine iodide/S-Butyrylthiocholine iodide, 2.76 mL Na2HPO4/NaH2PO4 buffer (pH 8.0, 0.1 mol/L), and 100 µL different concentrations of test compounds were incubated at 30 °C for 25 min. Then the reaction was terminated via adding 100 µL 20% sodium dodecyl sulfate (SDS), then 100 µL 10 mmol/L 5, 5'-Dithiobis-(2-nitrobenzoic acid) (DTNB) was added to generate the yellow anion 5-thio-2-nitro-benzoic acid. The absorbance of each assay mixture was measured at 412 nm by UV spectroscopy. The IC50 values were calculated by Bliss method and expressed as mean ± SD of the replicates. Rivastigmine was used as the control drug.
Kinetic studies
Kinetic experiment was conducted by a reported method with some modifications26. Compound 4l was added into the assay solution and pre-incubated with the enzyme at 30 °C, followed by the addition of 100-µL acetylthiocholine iodide including five concentrations. The assay solution contained 100 µL compound 4l, 100 µL DTNB, 2.76 mL 0.1 mol/L Na2HPO4/NaH2PO4 buffer (pH 8.0). Kinetic profile of AChE was determined by UV spectrophotometer at 412 nm. Additionally, the blank control experiment was conducted with the vehicle to replace compound 4l solution.
Molecular docking
Molecular docking was performed by molecular operating environment (MOE) software package (Chemical Computing Group Inc., Montréal, Canada). The X-ray crystallographic structures of AChE (PDB code: 1EVE) and BuChE (PDB code: 1P0I) were gained from protein data bank. 3D structure of compound 4l as the strongest AChE inhibitor in the present investigation was established by virtue of the builder interface of MOE program, and docked into the active site of the protein after energy being minimized. The dock scoring in MOE software was done by ASE scoring function.
Results and discussion
Chemistry
Three different chlorobenzaldehydes (compounds 2a–2c) reacted with 4-hydroxyl chalcone in ethanol with sodium hydroxide as catalyst to generate three 4'-hydroxy–chlorochalcones (compounds 3a–3c). Then compounds 3a–3c reacted with four different alkyl amines to gain a series of amine alkyl – substituted chlorochalcone derivatives (compounds 4a–4l) in the presence of potassium carbonate, acetone and sodium iodide. The total synthetic route is shown in Scheme 1.
Scheme 1.
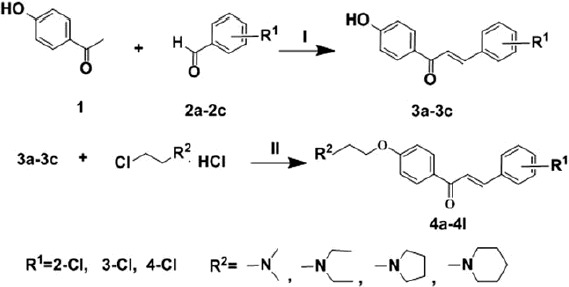
The synthesis of compounds 4a–4l (I) 30% NaOH, EtOH, 25 °C, stirring 36 h; (II) K2CO3, acetone, NaI, 56 °C, reflux.
For the synthesis of 4’-hydroxy–chlorochalcones, the concentration of sodium hydroxide dramatically influences the yield and purity of the products. As a result, 30% sodium hydroxide was chosen as the catalyst. For the synthesis of compounds 4a–4l, the solvent for the reaction is important. In this reaction, acetone was selected as the solvent for its easy operation and low toxicity. In addition, sodium iodide was used as the catalyst to enhance the reaction activity and accelerate the reaction process. For the synthesis of different tertiary amine substituted chlorochalcones, the reaction time ranged from 5 to 8 h. Pyrazoline compounds were synthesized from chlorochalcones, hydrazine and acetic acid (Scheme 2). High-yield desired compounds were gained via this reaction.
Scheme 2.
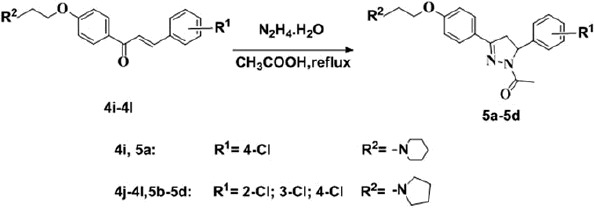
The synthesis of compounds 5a–5d.
New synthetic compounds were characterized by proton nuclear magnetic resonance spectroscopy (1H NMR), IR and mass spectrometry (MS) and elemental analysis. The purities of all synthesized compounds were confirmed to be higher than 98% by HPLC.
Bioactivity evaluation
Tertiary amine group, which is thought as a versatile pharmacophore appeared in the structures of many drugs in clinic practice, such as local anesthetics lidocaine, estrogen receptor modulator, tamoxifen, antipsychotic drug chlorpromazine and antimalarial chloroquine. Interestingly, in this investigation, some of synthesized compounds are known compounds. Compounds 3a and 3c were reported to be as anticancer agent and monoamine oxidase Inhibitor, respectively17,18. Compounds 4d and 4f were patent protected compounds three decades ago (US Patent 4342782, US Patent 2668813), but there are no reports on the bioactivity of them up to now20,21. Compounds 4i, 4j and 4l were reported as anticancer agents in pharmacology experiments22.
Although some known compounds contained in the present investigation, the bioactivity of them in inhibiting AChE was not be reported. As shown in Table 1, the data indicated that all amino alkyl substituted chlorochalcones exhibited better inhibitory activities against AChE than the precursor compounds 3a–3c (IC50 >500 µmol/L). Among them, compounds 4i, 4j, 4k and 4l with IC50 values of 0.91, 0.81, 0.28 and 0.17 µmol/L respectively, showed 11–62 folds inhibitory activity as that of Rivastigmine (IC50 = 10.54 µmol/L). In addition, compound 4l possessed high selectivity for AChE (Ratio: 667.2).
Table 1.
AChE and BuChE inhibitory activity and log p of chlorochalcone derivatives.
| IC50 (μmol/L)* |
||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | AChE | BuChE | Selectivity† | Log p |
| 3a | 2-Cl | – | >500 | >500 | – | – |
| 3b | 3-Cl | – | >500 | >500 | – | – |
| 3c | 4-Cl | – | >500 | >500 | – | – |
| 4a | 2-Cl | 2.11 ± 0.38 | 41.12 ± 2.31 | 19.5 | 1.75 | |
| 4b | 3-Cl | 3.76 ± 0.26 | 32.14 ± 7.16 | 8.5 | 1.83 | |
| 4c | 4-Cl | 1.55 ± 0.16 | 43.62 ± 6.94 | 28.1 | 1.61 | |
| 4d | 2-Cl | 5.42 ± 0.20 | 63.83 ± 9.21 | 11.8 | 1.63 | |
| 4e | 3-Cl | 5.83 ± 0.42 | 80.21 ± 7.18 | 13.8 | 1.62 | |
| 4f | 4-Cl | 3.78 ± 0.39 | 101.03 ± 17.23 | 26.7 | 1.69 | |
| 4g | 2-Cl | 1.80 ± 0.48 | 35.62 ± 7.31 | 19.8 | 1.75 | |
| 4h | 3-Cl | 1.31 ± 0.17 | 24.51 ± 6.14 | 18.7 | 1.72 | |
| 4i | 4-Cl | 0.91 ± 0.09 | 114.21 ± 26.15 | 125.5 | 1.79 | |
| 4j | 2-Cl | 0.81 ± 0.03 | 32.08 ± 5.71 | 39.6 | 1.85 | |
| 4k | 3-Cl | 0.28 ± 0.05 | 31.06 ± 3.41 | 110.9 | 1.81 | |
| 4l | 4-Cl | 0.17 ± 0.06 | 113.43 ± 18.22 | 667.2 | 1.83 | |
| 5a | 4-Cl | 5.94 ± 0.27 | 97.64 ± 3.78 | 16.4 | – | |
| 5b | 2-Cl | 6.31 ± 0.16 | 29.91 ± 1.25 | 4.74 | – | |
| 5c | 3-Cl | 2.41 ± 0.34 | 27.62 ± 2.58 | 11.5 | – | |
| 5d | 4-Cl | 2.04 ± 0.26 | 108.03 ± 5.56 | 52.96 | – | |
| Rivastigmine‡ | – | – | 10.54 ± 0.86 | 0.26 ± 0.08 | 0.025 | – |
IC50: 50% inhibitory concentration (means ± SD of three experiments).
Selectivity for AChE is defined as IC50 (BuChE)/IC50 (AChE).
Used for positive control.
Based on the data in Table 1, it seemed that the variation of amino-alkyl side chain markedly influence the inhibitory potency of compounds against AChE. Generally, the order of the inhibitory potency of these derivatives was as followed: pyrrolidine group > piperidine group > dimethylamine group > diethylamine group. Compounds 4j, 4k and 4l, which were substituted by pyrrolidine group, exhibited potent inhibitory activity with IC50 values less than 1.0 µmol/L. On other hand, the substituted position of chlorine atom in chalcone scaffold was very important for the inhibitory and selectivity for AChE. For dimethylamine or diethylamine substituted chlorochalcone derivatives the order of inhibitory potency against AChE was: Para > Meta > Ortho, while for piperidine or pyrrolidine substituted chlorochalcone derivatives the order was: Para > Orto > Meta. In addition, all Para-substituted chlorochalcone derivatives had the highest selectivity in inhibiting AChE over BuChE. Among them, compound 4l had the highest selectivity as 667 fold. Compared with the tertiary amine derivatives of fluorochalcone in our previous report, compound 4l showed higher selectivity against AChE than that of fluorochalcone derivatives27.
Compound 4l was selected for enzyme kinetic studies. The linear Lineweaver–Burk equation was applied to evaluate the inhibition profile. The graphical analysis of the steady-state inhibition data of compound 4l was shown in Supplement data: Figure 1. According to the analysis, compound 4l presented a mixed-type inhibition for that Km increased and Vmax decreased with the increasing of the concentration of compound 4l. The competitive inhibition constant (Ki) and the noncompetitive constant (Ki’) are 0.38 and 2.95 µmol/L, respectively (Supplement data: Table 2).
To explore the possible interacting mode between the chlorochalcone derivatives and AChE, molecular docking was performed for compound 4l with software MOE2008. As shown in Supplement data: Figure 2, this compound exhibited a multiple points-binding mode with AChE (Supplement data: Figure 2A). In the top of the gorge, the aromatic moiety adopted an appropriate orientation for its binding to peripheral anionic site (PAS), via the π–π stacking interaction with Trp279. The side chain interacted with Tyr334 in the mid-gorge zone. In the bottom of the gorge, the nitrogen of pyrrolidine ring binds to Trp84 via a cation–π in catalytic active site (CAS). Compared to the interaction between compound 4l and AChE mentioned above, compound 4l can only bind with BuChE via Tyr257 and Gly311 that were not the important amino acids in the catalytic process of BuChE (Supplement data: Figure 2B). These results may partially explain its potent inhibition and high selectivity for AChE. As a potential compound for treatment of AD, log p was thought as an important physical chemistry parameter to valuate or predict the ability to cross blood brain barrier (BBB). It was reported that the log p with optimum central nervous system (CNS) penetration was around 2 ± 0.728. As shown in Table 1, log p values of new synthesized compounds ranged from 1.61 to 1.83, which indicated that all the compounds had sufficiently lipophilicify to pass the BBB in vivo.
In further study to explore the influence of chalcone scaffold on bioactivity, pyrazoline derivatives were synthesized from chlorochalcones. The bioactivity evaluation showed that the inhibition potency of pyrazoline derivatives against AChE dramatically decreased compared to that of chlorochalcone derivatives (Table 1). It indicated that α,β-unsaturated ketone group in chalcone possibly pay an important role for the inhibitory activity against AChE. In addition, the selectivity of pyrazoline derivatives to inhibiting AChE over BuChE also decreased significantly.
Conclusion
In the present investigation, a series of chlorochalcones and pyrazoline derivatives were synthesized and evaluated in inhibiting AChE and BuChE. The result showed that the substituted position of chlorine significantly influenced the activity and selectivity of compounds in inhibiting AChE. For those compounds with the same amine alkyl side chain, Para-substituted chlorochalcone had the highest activity and selectivity. The pyrazoline derivatives synthesized from chlorochalcones with the cyclization of α,β-unsaturated ketone group had weaker activity and lower selectivity in inhibiting AChE compared to that of chlorochalcone derivatives, suggesting that α,β-unsaturated ketone group was important for inhibiting AChE. Among them, compound 4l revealed the strongest AChE inhibitory activity (IC50: 0.17 µmol/L) and highest selectivity (Ratio: 667.2). Enzyme kinetic study suggested that compound 4l was a mixed-type inhibitor. Molecular docking supported the mixed-type inhibition mechanism. Compound 4l might serve as a potential agent for the treatment of AD.
Disclosure statement
The authors confirm that this article content has no conflict of interest.
Funding
This study was financial supported by "The innovative investigation funds for post-graduated students of Hu’nan province" (No. CX2015424).
Supplementary Material
References
- 1.Pivtoraiko VN, Abrahamson EE, Leurgans SE, et al. Cortical pyroglutamate amyloid-β levels and cognitive decline in Alzheimer's disease. Neurobiol Aging 2015;36:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand R, Gill KD, Mahdi AA.. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 2014;76:27–50. [DOI] [PubMed] [Google Scholar]
- 3.García-Alberca JM.Cognitive intervention therapy as treatment for behavior disorders in Alzheimer disease: evidence on efficacy and neurobiological correlations. Neurología (English Edition) 2015;30:8–15. [DOI] [PubMed] [Google Scholar]
- 4.Small G, Bullock R. Defining optimal treatment with cholinesterase inhibitors in Alzheimer's disease. Alzheimers Dement 2011;7:177–84. [DOI] [PubMed] [Google Scholar]
- 5.Molinuevo JL, Gauthier S.. Benefits of combined cholinesterase inhibitor and memantine treatment in moderate-severe Alzheimer’s disease. Alzheimers Dement 2013;9:326–31. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee PK, Kumar V, Mal M, et al. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007;14:289–300. [DOI] [PubMed] [Google Scholar]
- 7.Li RS, Wang XB, Hu XJ, et al. Design, synthesis and evaluation of flavonoid derivatives as potential multifunctional acetylcholinesterase inhibitors against Alzheimer’s disease. Bioorg Med Chem Lett 2013;23:2636–41. [DOI] [PubMed] [Google Scholar]
- 8.Asadipour A, Alipour M, Jafari M, et al. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem 2013;70:623–30. [DOI] [PubMed] [Google Scholar]
- 9.Silva T, Reis J, Teixeira J, et al. Alzheimer's disease, enzyme targets and drug discovery struggles: from natural products to drug prototypes. Ageing Res Rev 2014;15:116–45. [DOI] [PubMed] [Google Scholar]
- 10.Liu HR, Huang XQ, Lou DH, et al. Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives flavokawain B. Bioorg Med Chem Lett 2014;24:4749–53. [DOI] [PubMed] [Google Scholar]
- 11.Liu HR, Liu XJ, Fan HQ, et al. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2014;22:6124–33. [DOI] [PubMed] [Google Scholar]
- 12.Liu HR, Fan HQ, Gao XH, et al. Design, synthesis and preliminary structure–activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: a further study based on Flavokawain B Mannich base derivatives. J Enzyme Inhib Med Chem 2016;3:580–9. [DOI] [PubMed] [Google Scholar]
- 13.Oztaşkın N, Cetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57. [DOI] [PubMed] [Google Scholar]
- 14.Aksu K, Topal F, Gulcin İ, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm 2015;348:446–55. [DOI] [PubMed] [Google Scholar]
- 15.Burmaoglu S, Algul O, Anil DA, et al. Synthesis and anti-proliferative activity of fluoro-substituted chalcones. Bioorg Med Chem Lett 2016;26:3172–6. [DOI] [PubMed] [Google Scholar]
- 16.Cole AL, Hossain S, Cole AM, et al. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg Med Chem 2016;24:2768–76. [DOI] [PubMed] [Google Scholar]
- 17.Syam S, Abdelwahab SI, Al-Mamary MA, et al. Synthesis of chalcones with anticancer activities. Molecules 2012;17:6179–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimenti F, Fioravanti R, Bolasco A, et al. Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem 2009;52:2818–24. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Shin MC, Yun YD, et al. Synthesis of aminoalkyl-substituted aurone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2015;23:231–40. [DOI] [PubMed] [Google Scholar]
- 20.Edward J, Cragoe J, Rahway NJ. 1-(Substituted-aminoalkoxyphenyl)-2-methylene-1-alkanones, compositions and their use. US 4342782[P] Aug 3, 1982. [Google Scholar]
- 21.Goldbeg WM, Montclair U, Rachlin AI, et al. Basic ketones and salts thereof. US 2668813[P] Feb 9, 1954. [Google Scholar]
- 22.Mokale SN, Dube PN, Bhavale SA, et al. Synthesis, in-vitro screening, and docking analysis of novel pyrrolidine and piperidine-substituted ethoxy chalcone as anticancer agents. Med Chem Res 2014;24:1842–56. [Google Scholar]
- 23.Insuasty B, Montoya A, Becerra D, et al. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur J Med Chem 2013;67:252–62. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Li WM, Kan KKW, et al. The physicochemical properties and the in vivo AChE inhibition of two potential anti-Alzheimer agents, bis(12)-hupyridone and bis(7)-tacrine. J Pharmaceut Biomed Anal 2008;46:75–81. [DOI] [PubMed] [Google Scholar]
- 25.Ellman GL, Courtney KD, Andres VJ, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95. [DOI] [PubMed] [Google Scholar]
- 26.Skrzypek A, Matysiak J, Niewiadomy A, et al. Synthesis and biological evaluation of 1,3,4-thiadiazole analogues as novel AChE and BuChE inhibitors. Eur J Med Chem 2013;62:311–19. [DOI] [PubMed] [Google Scholar]
- 27.Liu HR, Zhou C, Fan HQ, et al. Novel potent and selective acetylcholinesterase inhibitors as potential drugs for the treatment of Alzheimer's disease: synthesis, pharmacological evaluation, and molecular modeling of amino-alkyl-substituted fluoro-chalcones derivatives. Chem Biol Drug Des 2015;86:517–22. [DOI] [PubMed] [Google Scholar]
- 28.Glave WR, Hansch CJ.. Relationship between lipophilic character and anesthetic activity. J Pharm SCI-US 1972;61:589–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


