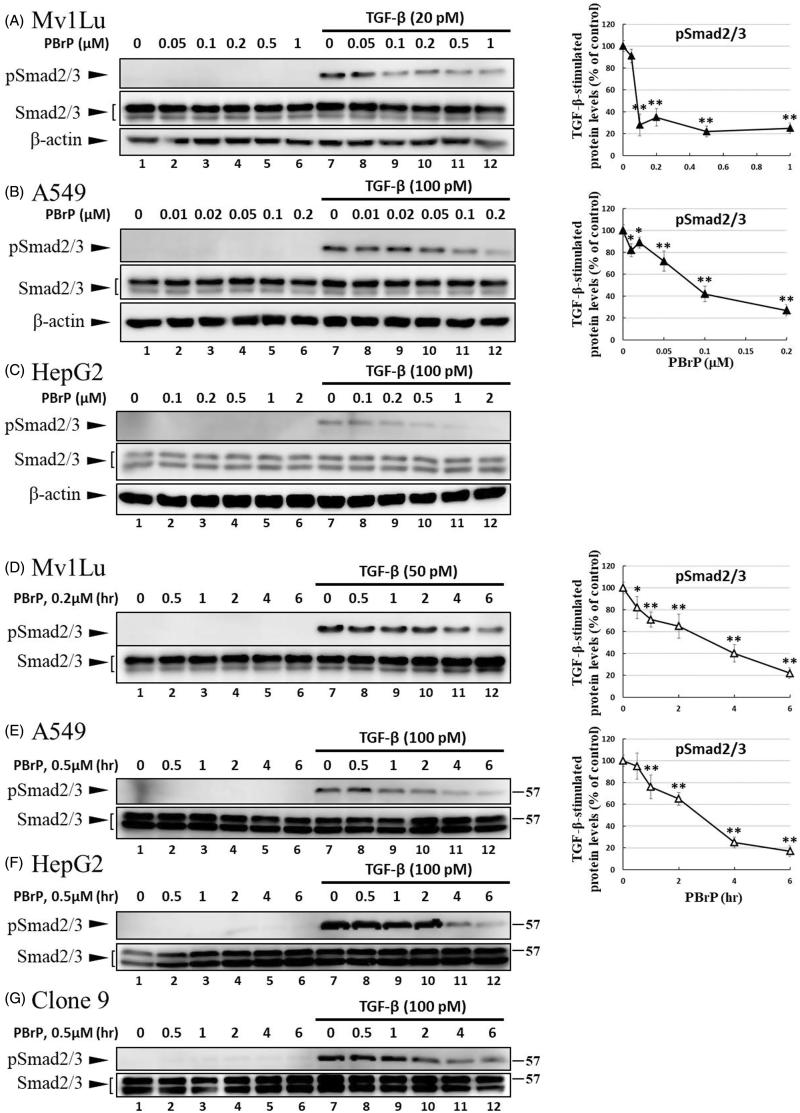
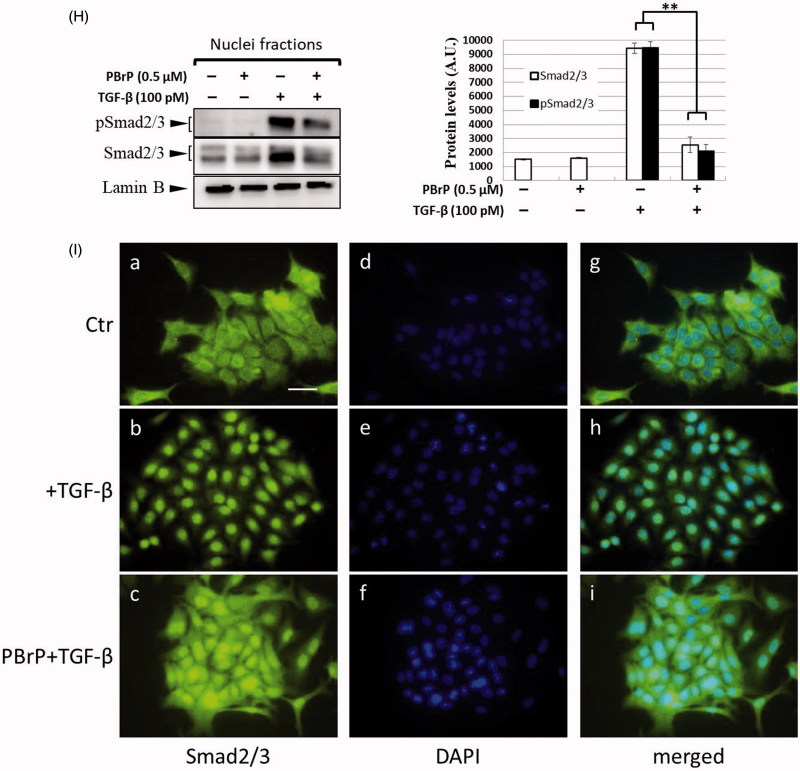
Figure 2.
PBrP inhibits TGF-β-stimulated Smad2/3 phosphorylation and nuclear translocation. Mv1Lu (A), A549 (B) and HepG2 (C) cells were pretreated with increasing concentrations (0.01–1 μM) of PBrP for 6 h followed by 30 min of TGF-β stimulation. Mv1Lu (D), A549 (E), HepG2 (F) and Clone 9 (G) cells were pretreated with 0.5 μM of PBrP for 0.5, 1, 2, 4 and 6 h followed by 30 min of TGF-β stimulation. Cell lysates were subjected to Western blot analysis with monoclonal antibodies against p-Smad2/3 and Smad2/3. An equal amount of protein loading was verified by Smad2/3 and β-actin (images were duplicated from the experiments illustrated in Figure 4). (H) Nuclear translocation of Smad proteins was analysed by separating nuclear fractions after treating A549 cells with TGF-β or PBrP for 6 h. Smad2/3 and pSmad2/3 proteins were detected through Western blot analysis using antibodies against Smad2/3 and p-Smad2/3. Complete fractionation of nuclear proteins and equivalent loading were verified through Western blot analysis using antibodies against Lamin B. Panels (A to H) show the representative images and right graphs illustrate quantitative analyses of ECL (mean ± SD) from at least three independent experiments (compare with TGF-β treatment alone); *p < .05, **p < .01. (I) Blockade of TGF-β-induced nuclear import of Smad2/3 by PBrP in A549 cells was determined through immunofluorescence. Cells were serum starved and pretreated with 0.5 μM of PBrP for 6 h followed by 30 min of TGF-β stimulation, and processed for immunofluorescence using the anti-Smad2/3 antibody. Fluorescence was visualised using the Alexa Fluor 488-conjugated donkey anti-goat antibody and a ZeissObserver fluorescence microscope. DAPI was used as a counterstain. Each experiment was repeated three times. Bar: 20 μm.