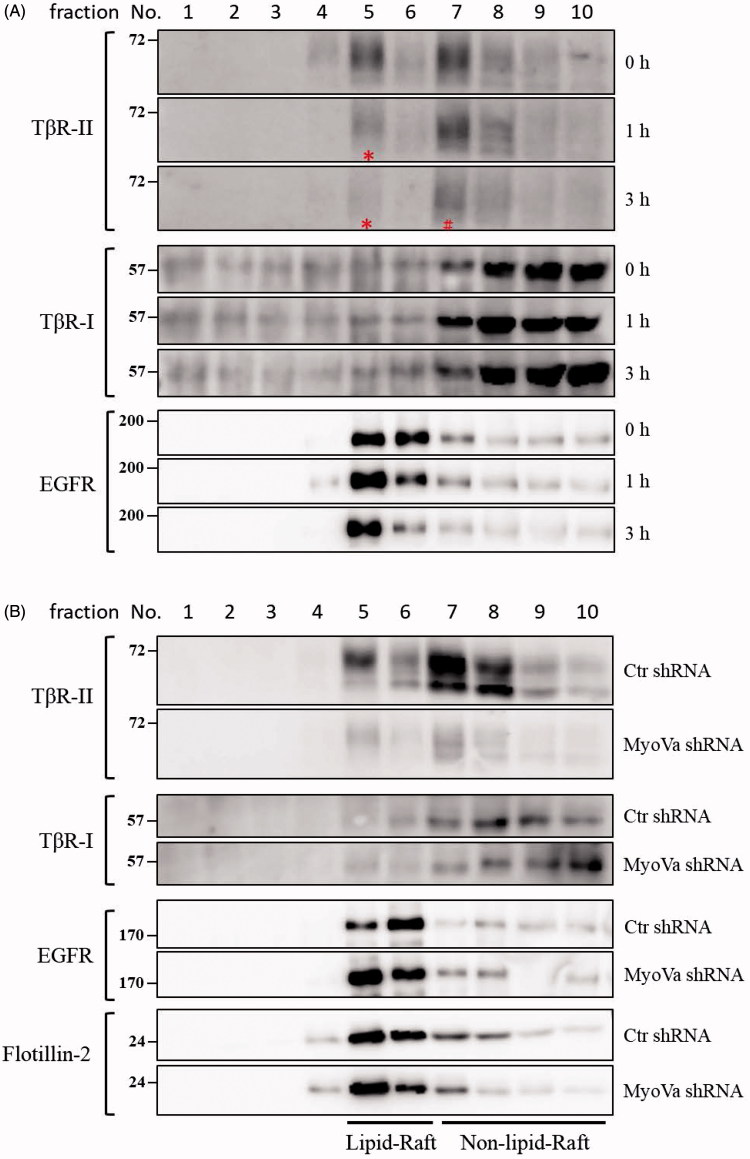
Figure 8.
PBrP induces TβRII degradation in lipid rafts. A549 cells or shRNA-silenced A549 cells were left untreated or incubated with 0.5 μM of PBrP for 0, 1 and 3 h in low-serum DMEM and lysed. Subsequently, 10 sucrose density gradient fractions of the lysates were collected through ultracentrifugation, as described in Materials and methods section. Thirty microgram of protein from each fraction was subjected to SDS-PAGE and transferred onto PVDF membranes, and blotted with anti-TβRII, anti-TβRI and EGFR antibodies. Protein samples from various treatments in acrylamide gel strips were synchronously electrotransferred onto the same PVDF membrane and then simultaneously proceeded for Western blotting; therefore, the signals of distinct proteins from various treatments were mutually comparable. (A) PBrP treatment reduced TβRII predominately in raft fractions within 3 h. (B) MyoVa depletion reduced TβRII in raft and non-raft fractions without altering the protein abundance and subcellular compartmentation of TβRI, EGFR or raft marker flotillin-2.