Abstract
The isatin scaffold is the constitutive fragment of several natural and synthetic bioactive molecules. Albeit several benzene sulphonamide-based carbonic anhydrase inhibitors (CAIs) have been reported, only recently isatin benzene sulphonamides have been studied and proposed as CAIs. In this study we have designed, synthesised, and evaluated the biological activity of a series of differently substituted isatin-based benzene sulphonamides which have been designed for the inhibition of carbonic anhydrase isoforms. The activity of all the synthesised compounds was evaluated towards human carbonic anhydrase I, II, IX, and XII isozymes. Our results indicate that the nature and position of substituents on the isatin ring can modulate both activity and isozyme selectivity.
Keywords: Antitumour agents, carbonic anhydrase inhibitors, isatin hybrid molecules
Introduction
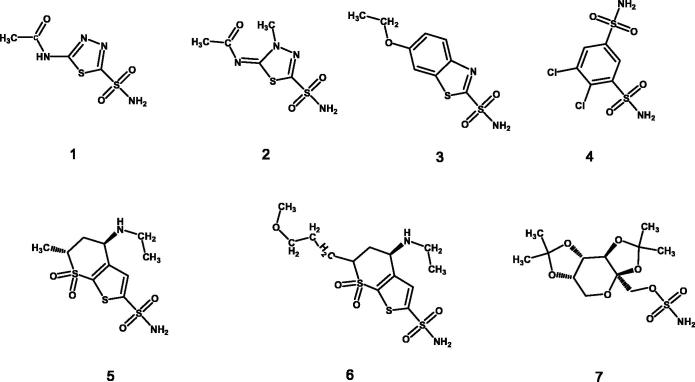
The isatin nucleus could be considered as a privileged scaffold for the design of biologically active agents. The discovery and optimisation of isatin-based therapeutic agents have consistently attracted the interest of medicinal chemists and the chemistry, the biological properties and the therapeutic potential of isatin-based agents has been recently reviewed1. Several biological activities could be achieved by decoration of the isatin scaffold such as anti-cancer2–7, anti-oxidant6, HIV reverse transcriptase inhibition8,9, neuroprotective10, anti-fungal11,12, anti-bacterial13,14 and anti-diabetic15. Moreover, the isatin ring has been pointed out as an essential part of anticancer hybrid molecules16,17. Recently, the design of isatin-based carbonic anhydrase inhibitors (CAIs) has been reported10,11,18–20. It is common knowledge that carbonic anhydrase isozyme family is involved in several physiological and/or pathological metabolic pathways21–26. They catalyse the simple reaction of the reversible hydration of carbon dioxide to bicarbonate and protons27, which is essential for the regulation of the different chemical species connected with CO2 in the body and its transport across biological membranes such as the inter-, intra- and extra-cellular spaces25,28. Not surprisingly several CAIs have been reported and their therapeutic potential has been directed towards different pathologies29–31 (Figure 1).
Figure 1.
Carbonic anhydrase inhibitors in clinical use: (1) acetazolamide, (2) (methazolamide, (3) ethoxzolamide, (4) dichlorphenamide, (5) dorzolamide, (6) brinzolamide, and (7) topiramate.
Within CAIs, benzene-sulphonamides are widely represented32–35and their binding on carbonic anhydrase investigated36. Benzene-sulphonamides are versatile scaffold that can be efficiently substituted to achieve isozyme specificity17,19,20,35,37–39. In this respect, the specific targeting of the tumour associated CA isoforms IX and XII represents an innovative and specific approach for the treatment of tumours23,40,41. Furthermore, the identification of hybrid molecules, containing both the isatin scaffold and the benzene-sulphonamide moiety, with a multi-pharmacological effect is an attractive target for medicinal chemists, for the treatment of multifactorial pathologies such as cancer41–43. On the basis of the above and with the aim to achieve structure-activity relationships on isatin derived CAIs, we have designed and synthesised a series of new 4-{[5–(2-oxo-2,3-dihydro-1H-indol-3-ylidene)-3-methyl-4-oxo-1,3-thiazolidin-2-ylidene]amino}benzene-1-sulphonamides as potential inhibitors of the tumour associated CA isoforms IX and XII.
Methods
Materials and apparatus
Starting materials and reagents were obtained from commercial suppliers and were used without purification. All melting points were determined on a Stuart SMP11 melting points apparatus (Stone, UK) and are uncorrected. Electron ionisation mass spectra were obtained by a Fisons QMD 1000 mass spectrometer (Danvers, MA) (70 eV, 200 mA, ion source temperature 200 °C). Samples were directly introduced into the ion source. Found mass values are in agreement with theoretical ones. Melting points, yield of reactions and the analytical data of derivatives are reported in Table 1.
Table 1.
Chemical, analytical, and physical data of derivatives EMAC 10020.
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | R.F.* | C--H--N |
M.P. °C | Yield % | Mass fragments | |
| Calc. | Found | ||||||
| EMAC 10020 a | H | 0.52 | C, 52.16; H, 3.40; N, 13.52 | C, 52.19; H, 3.39; N, 13.50 | 320d | 38 | 414; 386; 373 |
| EMAC 10020 c | 5-Cl | 0.38 | C, 48.16; H, 2.92; N, 12.48; | C, 48.19; H, 2.90; N, 12.46; | 320-1 | 71 | 448; 420; 407 |
| EMAC 10020 d | 5-F | 0.42 | C, 49.99; H, 3.03; N, 12.96; | C, 50.01; H, 3.00; N, 12.93; | 310-2 | 66 | 432; 404; 391 |
| EMAC 10020 l | 5-CH3 | 0.50 | C, 53.26; H, 3.76; N, 13.08; | C, 53.29; H, 3.73; N, 13.05; | 320-2 | 67 | 428; 400; 387 |
| EMAC 10020 m | 7-F | 0.65 | C, 49.99; H, 3.03; N, 12.96; | C, 50.00; H, 3.01; N, 12.95; | 288d | 43 | 432; 404; 391 |
| EMAC 10020 n | 5-CF3 | 0.51 | C, 47.30; H, 2.72; N, 11.61; | C, 47.33; H, 2.70; N, 11.59; | 314d | 41 | 482; 454; 441 |
| EMAC 10020 o | 7-Br | 0.69 | C, 43.82; H, 2.66; N, 11.36; | C, 43.85; H, 2.64; N, 11.33; | 320d | 75 | 492; 464; 451 |
R.F. values were obtained on silica gel plates using a mixture of ethyl acetate/n-hexane 2/1.
1H-NMR (Table 2) were registered on a Bruker 400 MHz spectrometer (Billerica, MA) or on a Varian 500 MHz (Palo Alto, CA) (Table 2). All samples were measured in DMSO. Chemical shifts are reported referenced to the solvent in which they were measured. Coupling constants J are expressed in hertz (Hz). Elemental analyses were obtained on a Perkin–Elmer 240 B microanalyser (Waltham, MA). Analytical data of the synthesised compounds are in agreement within ±0.4% of the theoretical values. TLC chromatography was performed using silica gel plates (Merck F 254, Billerica, MA), spots were visualised by UV light.
Table 2.
1H NMR and 13C NMR data of derivatives EMAC 10020.
| Compound | 1H NMR δ (ppm) |
|---|---|
| EMAC 10020 a | 1H NMR (500 MHz, DMSO-d6) δ (ppm): 3.32- 3.36 (3H, s, CH3, N-CH3); 7.05- 7.09 (1H, s, CH, Isat.); 6.91-6.93 (1H, d, J = 7.6 Hz, CH, Isat.); 7.20 (2H, d, CH, J = 8.8 Hz, 4-SO2NH2 phenyl); 7.35- 7.39 (3H, m, CH + NH2, Isat.+ SO2NH2)7.86- 7.89 (2H, d, CH, J = 8.8 Hz, 4-SO2NH2 phenyl); 8.84- 8.86 (1H, d, CH, J = 8 Hz, Isat.); 11.20 (1H, s, NH, Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 29.10, 109.93, 120.10, 121.46, 123.55, 127.19, 128.05, 124.30, 130.80, 132.37, 140.16, 140.98, 150.52, 154.32, 165.46, 168.40. |
| EMAC 10020 c | 1H NMR (500 MHz, DMSO-d6) δ (ppm): 3.29- 3.37 (3H, s, CH3, N-CH3); 6.93- 6.95 (1H, d, CH, J = 8.5 Hz, 5-Cl Isat.); 7.16- 7.20 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 7.33 (2H, s, NH2, SO2NH2); 7.41- 7.43 (1H, d, CH, Jo = 8.5 Hz, Jm= 2, 5 Hz -Cl Isat.); 7.86- 7.88 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 8,89 (1H, d, CH, J = 2 Hz, 5-Cl Isat.); 11.32 (1H, s, NH, 5-Cl Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 28.88, 111.47, 121.23, 121.41, 123.79, 125.81, 127.10, 127.31, 131.08, 132.55, 140.32, 141.89, 150.32, 153.96, 165.44, 168.05. |
| EMAC 10020 d | 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.33 (3H, s, CH3, N-CH3); 6.88 (1H, m, CH, 5-F Isat.); 7.18- 7.22 (2H, d, CH, J = 8.4 Hz, 4-SO2NH2 phenyl); 7.22-7.25 (1H, d, CH, 5-F Isat.); 7.37 (2H, s, NH2, SO2NH2); 7.87- 7.90 (2H, d, CH, J = 8.8 Hz, 4-SO2NH2 phenyl); 8.58 (1H, m, CH, 5-F Isat.); 11.20 (1H, s, NH, 5-F Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 28.91, 111.08, 114.54, 118.16, 120.70, 121.38, 124.46, 127.28, 132.35, 140.34, 150.35, 154.06, 156.30, 158.63, 165.58, 168.35. |
| EMAC 10020 l | 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.30 (3H, s, CH3, 5-CH3 Isat.); 3.30- 3.35 (3H, s, CH3, N-CH3); 6.79 (1H, d, CH, 5-CH3 Isat.); 7.16-7.19 (3H, m, CH, 4-SO2NH2 phenyl +5-CH3 Isat); 7.33 (2H, s, NH2, SO2NH2); 7.87 (2H, d, CH, J = 8 Hz, 4-SO2NH2 phenyl); 8.67 (1H, s, CH, 5-CH3 Isat.); 11.05 (1H, s, NH, 5-CH3 Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 21.14, 29.12, 109.94, 120.06, 121.47, 123.50, 127.20, 128.03, 130.12, 130.70, 132.36, 140.15, 140.97, 150.52, 154.32, 165.45, 168.39. |
| EMAC 10020 m | 1H NMR (500 MHz, Acetone-d6) δ (ppm): 3.48 (3H, s, CH3, N-CH3); 7.13-7.15(1H, m, 7-F Isat.); 7.21-7.23 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 7.25-7.29 (3H, m, CH + NH2,7-F Isat.+ SO2NH2), 7.96 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 8.85 (1H, d, CH, J = 8 Hz, 7-F Isat.); 10.43 (1H, s, NH, 7-F Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 28.91, 111.10, 114.54, 118.16, 120.70, 121.38, 122.90, 125.90, 127.28, 132.35, 150.35, 154.06, 156.30, 162.90, 165.58, 168.35. |
| EMAC 10020 n | 1H NMR (400 MHz, Acetone-d6) δ (ppm): 3.35 (3H, s, CH3, N-CH3); 7.04- 7.08 (1H, m, CH, 5-CF3 Isat.); 7.12 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 7.27- 7.31 (1H, t, CH, J = 8 Hz, 5-CF3 Isat.); 7.31 (2H, weak s, NH2, SO2NH2); 7.99 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 8.67 (1H, d, CH, J = 8 Hz, 5-CF3 Isat.); 11.67 (1H, NH, 5-CF3 Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 29.13, 109.95, 120.07, 121.47, 123.50, 124.60, 127.20, 128.04, 130.12, 130.71, 132.36, 140.16, 140.97, 150.53, 154.32, 165.45, 168.39. |
| EMAC 10020 o | 1H NMR (500 MHz, DMSO-d6) δ (ppm): 3.34 (3H, s, CH3, N-CH3); 6.99- 7.02 (1H, t, CH, J = 8 Hz, 7-Br Isat); 7.19- 7.20 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 7.34 (2H, s, NH2, SO2NH2); 7.54- 7.55 (1H, d, J = 8 Hz, CH, 7-Br Isat.); 7.87- 7.89 (2H, d, CH, J = 8.5 Hz, 4-SO2NH2 phenyl); 8.81- 8.83 (1H, d, CH, J = 8 Hz, 7-Br. Isat.); 11.43 (1H, s, NH, 7-Br Isat.). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 29.02, 102.69, 121.45, 121.68, 123.37, 124.44, 126.71, 127.26, 132.79, 134.27, 140.30, 142.17, 150.38, 153.97, 165.31, 168.26. |
Biological activity
Carbonic anhydrase inhibition assay
The purification of cytosolic CA isoenzymes (CA I and CA II) were previously described with a simple one-step method by a Sepharose-4B-L tyrosine-sulphanilamide affinity chromatography44. The protein quantity in the column effluents was determined spectrophotometrically at 280 nm. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied with a Bio-Rad Mini Gel system Mini-PROTEIN® system (Hercules, CA), Bio-Rad Laboratories, Inc., China after purification of both CA isoenzymes. Briefly, it was performed in acrylamide for the running (10%) and the stacking gel (3%) contained SDS (0.1%), respectively. Activities of CA isoenzymes were determined according to a method by Verporte et al.45 The increase in absorbance of reaction medium was spectrophotometrically recorded at 348 nm. Also, the quantity of protein was determined at 595 nm according to the Bradford method46. Bovine serum albumin was used as standard protein. The IC50 values were obtained from activity (%) versus compounds plots47. For calculation of KI values, three different concentrations were used. The Lineweaver–Burk curves were drawn and calculations were realised47. The biological data are reported in Table 3.
Table 3.
Inhibition data towards hCA I, II, IX, and XII of compounds EMAC 10020.
| KI (nM) | |||||
|---|---|---|---|---|---|
| Compound | Structure | hCA I | hCA II | hCA IX | hCA XII |
| EMAC 10020 a |  |
95.5 | 40.7 | 40.1 | 841 |
| EMAC 10020 c | 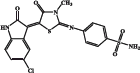 |
958 | 8300 | 55.7 | 865 |
| EMAC 10020 d | 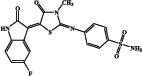 |
50.3 | 274 | 50.7 | 85.0 |
| EMAC 10020 l | 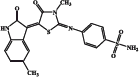 |
87.6 | 79.1 | 3.0 | 440 |
| EMAC 10020 m | 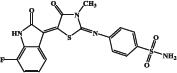 |
44.3 | 139 | 4.1 | 90.7 |
| EMAC 10020 n | 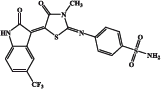 |
945 | 5720 | 2660 | 873 |
| EMAC 10020 o | 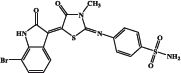 |
877 | 8780 | 126 | 824 |
| Acetazolamide | 250 | 12 | 25 | 5.7 | |
General procedure for the synthesis of compound EMAC10020
Synthesis of 1-methyl-3–(4-sulphamoylphenyl)thiourea1
4-Aminobenzensulphonamide (1 eq.) was refluxed in 2-propanol until a clear solution is obtained. Then a solution of methylisothiocyanate (1 eq.) in 2-propanol was added dropwise. By adding the isothiocyanate the solution became yellowish. The mixture was stirred until reaction completion (5–6 h) monitored by TLC (ethyl acetate/n-hexane 2/1). The reaction is allowed to cool down at r.t. and the formation of a white foaming precipitate is observed, which was filtered and crystallised from ethanol. White crystals; MW: 245.32 g/mol; yield: 65%; Mp: °C 204–6
1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.15 (3H, s, CH3, N-CH3); 7.08 (d, 2H, CH, J = 8.4, 4-SO2NH2 phenyl); 7.30 (2H, s, NH2, SO2NH2); 7.80 (d, 2H, CH, J = 8.4, 4-SO2NH2 phenyl); 10.01 (s, 2H,NH, thiourea).
Synthesis of 4-(4-oxo-1,3-thiazolidin-2-ylidene)aminobenzene-1-sulphonamide2
An ethanol solution of 1 (1.70 eq.), ethyl bromoacetate (1.90 eq) and anhydrous sodium acetate (6.9 eq.) was refluxed under vigorous stirring till the completion of the reaction (16–20 h), TLC (ethyl acetate/n-hexane 2/1). Then the solution was cooled to 0 °C, and the formed precipitate filtered under vacuum and crystallised from water.
White powder; MW: 285.34 g/mol; yield: 86%; Mp: 179–180 °C
1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.17 (3H, s, CH3, N-CH3); 4.06 (s, 2H, CH2, thiazol.); 7.10 (d, 2H, CH, J = 8.4, 4-SO2NH2 phenyl); 7.31 (2H, s, NH2, SO2NH2); 7.80 (d, 2H, CH, J = 8.4, 4-SO2NH2 phenyl).
Synthesis of 4-(3-methyl-4-oxo-5-(2-oxoindolin-3-ylidene)thiazolidin-2-ylideneamino)benzene-sulphonamides EMAC 10020
A mixture of 4-(3-methyl-4-oxothiazolidin-2-ylideneamino)benzene-sulphonamide (1 eq), the opportune isatin derivative (1 eq), acetic anhydride (1,5 eq), and sodium acetate (2 eq) was refluxed overnight in acetic acid. Once the reaction has come to completion TLC (ethyl acetate/n-hexane 2/1) the hot suspension was filtered. The obtained red/orange solid was washed with water. Compounds EMAC 10020 were purified by column chromatography on silica gel (ethyl acetate/n-hexane 2/1) to obtain the desired compounds whose data are reported in Tables 1 and 2.
Results and discussion
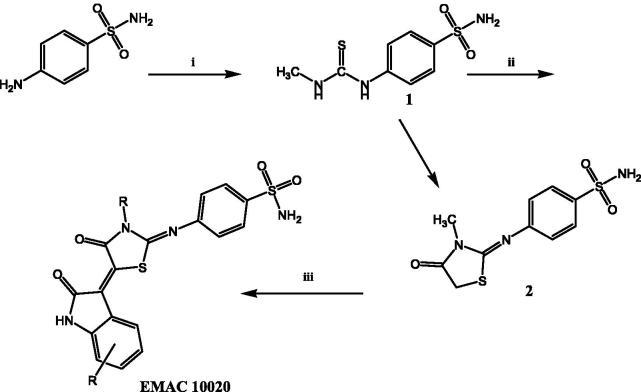
As a part of our ongoing research in the field of CAIs37 and to achieve a better understanding of the structural requirements for the selective inhibition of the different CA isoforms, we have synthesised a series of 4-(3-methyl-4-oxo-5-(2-oxoindolin-3-ylidene)thiazolidin-2-ylideneamino)-benzenesulphonamides indicated as compounds EMAC 10020 a, c, d, l, m, n, and o. All the synthesised compounds bear a differently substituted isatin scaffold linked, by the interposition of a thiazolidinone spacer, to a benzene-sulphonamide moiety, as zinc binder group. The synthesis of compounds EMAC 10020 was performed as illustrated in Figure 2.
Figure 2.
Synthetic pathway to compounds EMAC 10020. Reagents and conditions: (i) 2-propanol, methyl isothiocyanate; (ii) ethanol, ethyl bromoacetate, dry sodium acetate; (iii) R-isatin, acetic anhydride, dry sodium acetate, acetic acid.
The procedure consists of two steps. The first step is the synthesis of the 4-sulphamoylphenyl-thiourea derivative (1 of Figure 2) by simple reaction of the 4-aminobenzensulphonamide with methylisothiocyanate. The second step of the synthetic route consists of the formation of the thiazolidinone spacer2 which can be obtained by reacting 1 with ethyl-bromoacetate. Desired compounds were obtained by reacting compound 2 with the appropriate isatin. We attempted to perform step two and three in a one step one pot reaction, but our efforts only gave poor yields with respect to the three step procedure which was therefore preferred. Compounds EMAC 10020 were submitted to enzymatic assay to evaluate their activity and selectivity towards human CA (hCA) isozymes I, II, IX, XII. The results are illustrated in Table 3. As shown in Table 3 some of the tested compounds could be considered as hCA IX preferential inhibitors. However, the nature and position of the substituents on the isatin scaffold played a crucial role in determining the activity and the isozyme selectivity. In the case of compound EMAC 10020 a, bearing an un-substituted isatin, almost no selectivity can be observed, but for a very poor activity towards XII isozyme. With respect to isozyme IX, the introduction of an electron withdrawing (EW) group (compounds EMAC 10020 c, EMAC 10020 d, and EMAC 10020 n) in the position 5 of the isatin lead to a decrease of activity, albeit some selectivity towards hCA IX could be observed for compound EMAC 10020 c. However, the isosteric replacement of the chlorine atom and of the trifluoro-methyl moiety by a methyl group lead to an increase of activity (KI 3.0 nM) and selectivity. According to these preliminary results, in the case hCA IX, the presence of an EW group in the position 5 of the isatin is not tolerated while the introduction of an electron donating substituent in the same position is beneficial for the activity and selectivity. Also in the case of compound EMAC 10020 m, bearing a fluorine atom in the position 7 of the isatin ring, a good activity (KI 4.1 nM) and selectivity towards hCA IX was observed. Conversely the introduction of a bulkier EW group in the position 7 of the isatin scaffold, as for compound EMAC 10020 o, leads to a decrease of activity towards all isozymes. All together these data corroborate the hypothesis that the hybridisation of isatin scaffold with the benzene-sulphonamide moiety could be advantageous for the design of new therapeutic agents targeting the tumour associated hCA IX isoform.
Conclusions
We have synthesised a small library of isatin/benzene-sulphonamides hybrids for the inhibition of hCA isoforms. The activity of the newly synthesised derivatives has been evaluated towards hCA I, II, IX, and XII. Compounds EMAC 10020 c, EMAC 10020 l, and EMAC 10020 m could be considered as preferential hCA IX inhibitors. Our data indicated that the nature and the position of the substituents play a crucial role in tuning activity and selectivity towards hCA isozyme. Overall these data support the hypothesis that isatin hybrid molecules could represent a valuable starting point for the design of active and selective hCAIs.
Acknowledgements
The authors wish to acknowledge the “Ufficio Valorizzazione dei Risultati della Ricerca” of Sardegna Ricerche Technological Park, Pula (CA) – Italy.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Rane RA, Karunanidhi S, Jain K, et al. . A recent perspective on discovery and development of diverse therapeutic agents inspired from isatin alkaloids. Curr Top Med Chem (Sharjah, United Arab Emirates) 2016;16:1262–89. [DOI] [PubMed] [Google Scholar]
- 2.Hou J, Jin K, Li J, et al. . LJNK, an indoline-2,3-dione-based aminopeptidase N inhibitor with promising antitumor potency. Anti-Cancer Drugs 2016;27:496–507. [DOI] [PubMed] [Google Scholar]
- 3.Pape VFS, Toth S, Furedi A, et al. . Design, synthesis and biological evaluation of thiosemicarbazones, hydrazinobenzothiazoles and arylhydrazones as anticancer agents with a potential to overcome multidrug resistance. Eur J Med Chem 2016;117:335–54. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Ning Y, Zhao W, et al. . Synthesis, neuro-protection and anti-cancer activities of simple isatin mannich and schiff bases. Lett Drug Des Discov 2016;13:395–400. [Google Scholar]
- 5.Rana S, Blowers EC, Tebbe C, et al. . Isatin derived spirocyclic analogues with α-methylene-γ-butyrolactone as anticancer agents: a structure-activity relationship study. J Med Chem 2016;59:5121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Premanathan M, Radhakrishnan S, Kulangiappar K, et al. . Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl. Indian J Med Res 2012;136:822–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Pettersson M, ed. Sunitinib (Sutent): an angiogenesis inhibitor. Hoboken (NJ): John Wiley & Sons, Inc; 2010. [Google Scholar]
- 8.Corona A, Meleddu R, Esposito F, et al. . Ribonuclease H/DNA polymerase HIV-1 reverse transcriptase dual inhibitor: mechanistic studies on the allosteric mode of action of isatin-based compound RMNC6. PLoS One 2016;11:e0147225/1-e/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meleddu R, Distinto S, Corona A, et al. . (3Z)-3-(2-[4-(aryl)-1,3-thiazol-2-yl]hydrazin-1-ylidene)-2,3-dihydro-1H-indol-2-one derivatives as dual inhibitors of HIV-1 reverse transcriptase. Eur J Med Chem 2015;93:452–60. [DOI] [PubMed] [Google Scholar]
- 10.Tavari M, Malan SF, Joubert J.. Design, synthesis, biological evaluation and docking studies of sulphonyl isatin derivatives as monoamine oxidase and caspase-3 inhibitors. MedChemComm 2016. [Epub ahead of print]. doi: 10.1039/C6MD00228E. [DOI] [Google Scholar]
- 11.Akdemir A, Guzel-Akdemir O, Karali N, Supuran CT. Isatin analogs as novel inhibitors of Candida spp. β-carbonic anhydrase enzymes. Bioorg Med Chem 2016;24:1648–52. [DOI] [PubMed] [Google Scholar]
- 12.Chundawat TS, Kumari P, Sharma N, Bhagat S.. Strategic synthesis and in vitro antimicrobial evaluation of novel difluoromethylated 1-(1, 3-diphenyl-1H-pyrazol-4-yl)-3, 3-difluoro-1, 3-dihydro-indol-2-ones. Med Chem Res 2016. [Epub ahead of print]. doi: 10.1007/s00044-016-1658-z. [DOI] [Google Scholar]
- 13.Sahoo S, Mahendrakumar CB, Setty CM.. Synthesis, antiinflammatory and antibacterial activities of some substituted isatin and isatin fused with 3-substituted 4-amino-5-mercapto-1, 2, 4-triazoles. Int J Chem Sci 2015;13:613–24. [Google Scholar]
- 14.Roopan SM, Khan FRN, Selvan NT.. Synthesis of 1-[(2-chloroquinolin-3-yl)methyl]indoline-2,3-dione derivatives as potential antimicrobials. J Pharm Res 2010;3:950–2. [Google Scholar]
- 15.Wang G, Peng Z, He D, Yan C, Liu W, inventors; Jishou University, Peop. Rep. China. assignee. Coumarin-isatin type compound useful in treatment of diabetes mellitus and its preparation patent CN105237521A; 2016. [Google Scholar]
- 16.Havrylyuk D, Zimenkovsky B, Vasylenko O, et al. . Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J Med Chem 2012;55:8630–41. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim HS, Abou-Seri SM, Tanc M, et al. . Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumour-associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 2015;103:583–93. [DOI] [PubMed] [Google Scholar]
- 18.Ozgun DO, Yamali C, Gul HI, Taslimi P, Gulcin I, Yanik T, et al. . Inhibitory effects of isatin Mannich bases on carbonic anhydrases, acetylcholinesterase, and butyrylcholinesterase. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2016.1149479. [DOI] [PubMed] [Google Scholar]
- 19.Eldehna WM, Fares M, Ceruso M, et al. . Amido/ureidosubstituted benzenesulfonamides-isatin conjugates as low nanomolar/subnanomolar inhibitors of the tumour-associated carbonic anhydrase isoform XII. Eur J Med Chem 2016;110:259–66. [DOI] [PubMed] [Google Scholar]
- 20.Guzel-Akdemir O, Akdemir A, Karali N, Supuran CT.. Discovery of novel isatin-based sulphonamides with potent and selective inhibition of the tumour-associated carbonic anhydrase isoforms IX and XII. Org Biomol Chem 2015;13:6493–9. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Mokhtari RB, Pan J, et al. . Carbonic anhydrase II mediates malignant behavior of pulmonary neuroendocrine tumors. Am J Respir Cell Mol Biol 2015;52:183–92. [DOI] [PubMed] [Google Scholar]
- 22.Imtaiyaz Hassan M, Shajee B, Waheed A, et al. . Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 2013;21:1570–82. [DOI] [PubMed] [Google Scholar]
- 23.Supuran CT.Inhibition of carbonic anhydrase IX as a novel anticancer mechanism. World J Clin Oncol 2012;3:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL.. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 2010;29:6509–21. [DOI] [PubMed] [Google Scholar]
- 25.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3-related species. Biochim Biophys Acta 2010;1804:410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 2007;26:299–310. [DOI] [PubMed] [Google Scholar]
- 27.Domsic JF, Avvaru BS, Kim CU, et al. . Entrapment of carbon dioxide in the active site of carbonic anhydrase II. J Biol Chem 2008;283:30766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geers C, Gros G.. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 2000;80:681–715. [DOI] [PubMed] [Google Scholar]
- 29.Supuran CT.Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. [DOI] [PubMed] [Google Scholar]
- 30.Supuran CT.Carbonic anhydrase inhibitors in the treatment and prophylaxis of obesity. Expert Opin Ther Pat 2003;13:1545–50. [Google Scholar]
- 31.Picard F.Topiramate reduces energy and fat gains in lean (Fa/?) and obese (fa/fa) Zucker rats . Obes Res 2000;8:656–63. [DOI] [PubMed] [Google Scholar]
- 32.Carta F, Scozzafava A, Supuran CT.. Sulphonamides: a patent review (2008-2012). Expert Opin Ther Pat 2012;22:747–58. [DOI] [PubMed] [Google Scholar]
- 33.Suthar SK, Bansal S, Lohan S, et al. . Design and synthesis of novel 4-(4-oxo-2-arylthiazolidin-3-yl)benzenesulfonamides as selective inhibitors of carbonic anhydrase IX over I and II with potential anticancer activity. Eur J Med Chem 2013;66:372–9. [DOI] [PubMed] [Google Scholar]
- 34.Supuran Claudiu T.Structure and function of carbonic anhydrases . Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 35.Pala N, Micheletto L, Sechi M, et al. . Carbonic anhydrase inhibition with benzenesulfonamides and tetrafluorobenzenesulfonamides obtained via click chemistry. ACS Med Chem Lett 2014;5:927–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alterio V, Di Fiore A, D'Ambrosio K, et al. . Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 37.Meleddu R, Maccioni E, Distinto S, et al. . New 4-[(3-cyclohexyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene) amino]benzene-1-sulphonamides, synthesis and inhibitory activity toward carbonic anhydrase I, II, IX, XII. Bioorganic Med Chem Lett 2015;25:3281–4. [DOI] [PubMed] [Google Scholar]
- 38.Grandane A, Tanc M, Di Cesare Mannelli L, et al. . 6-Substituted sulfocoumarins are selective carbonic anhydrase IX and XII inhibitors with significant cytotoxicity against colorectal cancer cells. J Med Chem 2015;58:3975–83. [DOI] [PubMed] [Google Scholar]
- 39.Bozdag M, Ferraroni M, Nuti E, et al. . Combining the tail and the ring approaches for obtaining potent and isoform-selective carbonic anhydrase inhibitors: solution and X-ray crystallographic studies. Bioorg Med Chem 2014;22:334–40. [DOI] [PubMed] [Google Scholar]
- 40.Supuran CT, Winum J-Y.. Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med Chem 2015;7:1407–14. [DOI] [PubMed] [Google Scholar]
- 41.De Monte C, Carradori S, Gentili A, et al. . Dual cyclooxygenase and carbonic anhydrase inhibition by nonsteroidal anti-inflammatory drugs for the treatment of cancer. Curr Med Chem 2015;22:2812–18. [DOI] [PubMed] [Google Scholar]
- 42.Anighoro A, Bajorath J, Rastelli G.. Polypharmacology: challenges and opportunities in drug discovery. J Med Chem 2014;57:7874–87. [DOI] [PubMed] [Google Scholar]
- 43.Rosini M.Polypharmacology: the rise of multitarget drugs over combination therapies. Future Med Chem 2014;6:485–7. [DOI] [PubMed] [Google Scholar]
- 44.Akbaba Y, Akincioglu A, Gocer H, et al. . Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42. [DOI] [PubMed] [Google Scholar]
- 45.Verpoorte JA, Mehta S, Edsall JT.. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9. [PubMed] [Google Scholar]
- 46.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 47.Senturk M, Gulcin I, Beydemir S, et al. . In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9. [DOI] [PubMed] [Google Scholar]