Abstract
Retinal diseases, including age-related macular degeneration (AMD) and diabetic retinopathy (DR) are the leading causes of blindness in adults over the age of 50 years in the US. While most of those conditions do not have a cure, currently available treatment options attempt to prevent further vision loss. For many ophthalmic drugs, an efficient delivery system to provide maximum therapeutic efficacy and promote patient compliance remains an unmet medical need. An exploration of literature via PubMed spanning from 2007 to 2017 was conducted to identify studies that have evaluated nanotechnology as platforms for delivering therapeutic agents to the posterior segment of the eye where the retina is located. Until now, four routes that have been utilized for retinal drug delivery are the intravitreal, periocular, subretinal, and systemic routes. Intravitreal injections are now widely used in clinical practice due to their ability to directly target the back of the eye but are highly invasive procedures that may cause several complications, particularly with repeated uses over a short timespan. Nanotechnology shows great promise to revolutionize retinal drug delivery, offering many advantages such as a targeted delivery system towards the specific site of the retina as well as sustained delivery of therapeutic agents. In this review, specific eye anatomy and constraints on ocular drug administration are illustrated. Further, we list and highlight several examples of nanosystems, such as hydrogels, liposomes, dendrimers, and micelles, used via different drug delivery routes to treat various retinal diseases.
Keywords: nanotechnology, nanoparticle, drug delivery, retina, age-related macular degeneration, diabetic retinopathy
INTRODUCTION
The human eye is vulnerable to different types of damage, whether genetic or acquired. The eye's structure can be classified into two parts: 1) the anterior segment; 2) the posterior segment. The pupil, cornea, iris, ciliary body, aqueous humor, and lens make up the anterior segment, whereas vitreous humor, macula, retina, choroid, and optic nerve are parts of the posterior segment. The retina is the nerve layer that receives light, which creates impulses that are transmitted through the optic nerve to the brain. The macula is a small area of the retina that contains special light-sensitive cells[1]. Any dysfunction or deterioration of vital ocular tissue often has a profound impact on patient quality of life. The most common retinal diseases include age-related macular degeneration (AMD) and diabetic retinopathy (DR). Cytomegalovirus (CMV) retinitis, proliferative vitreoretinopathy, Stargardt disease and retinoblastoma also occur but are relatively rare in the US.
According to a report from the United States Center for Disease Control (CDC), it is estimated that more than 3.3 million Americans, aged 40 years and older, are either legally blind or have poor vision. In the US, AMD is the leading cause of permanent impairment of vision among people aged 65 years and older, while DR is mainly responsible for blindness among young to middle-aged individuals[2]. Over the past decades, a better understanding of the molecular mechanisms of ocular pathogenesis has led to the development of several new therapeutic entities. However, how to adequately deliver therapeutic agents to the back of the eye (i.e. the retina) remains a challenge. Intravitreal injections are now the standard method to administer retinal drugs, but associated complications are always a concern. Improving the efficacy of intravitreal injections and increasing patient compliance have gained the most research interest accompanied by ongoing research to explore the feasibility of periocular and systemic routes for retinal drug delivery. Owing to the advance of material and bioengineering science, nanotechnology has become one of the fastest growing fields today. A wide range of innovations in this area holds promise for more effective ocular drug delivery systems. In this review, specific eye anatomy and constraints in ocular drug administration are illustrated. Second, several examples of nanoparticles reported in the past ten years for management of ocular diseases are highlighted and reviewed, followed by our perspective on opportunities and challenges for future nanotechnology in retinal drug delivery. However, this mini-review is intended to be a nanotechnology introduction rather than a compilation of all reported nanoparticles. For a comprehensive summary of nanoparticles for retinal drug delivery, we refer the reader to the publications by Bisht et al[3], Joseph and Venkatraman[4].
TREATMENTS FOR RETINAL DISEASES AND OCULAR BARRIERS TO RETINAL DRUG DELIVERY
Currently, a number of therapeutic agents are available to treat retinal diseases, which include monoclonal antibodies (e.g. bevacizumab), fusion proteins (e.g. aflibercept) and small molecules (e.g. ganciclovir). Compared with small molecules, antibodies typically display a prolonged half-life, higher specificity to their sites of action and decreased toxicity[5]. The anti-vascular endothelial growth factor (anti-VEGF) drugs, e.g. bevacizumab (Avastin®; Genentech, Inc., CA, USA), ranibizumab (Lucentis®; Genentech, Inc., CA, USA) and aflibercept (Eylea®; Regeneron Pharmaceuticals, Inc., NY, USA), are widely used in the US to treat “wet” (neovascular) AMD over the last decade[6]. Regarding DR, a retinal disease caused by complications of diabetes, in 2015 US Food and Drug Administration (FDA) approved aflibercept to be used for this condition, following aflibercept's earlier approval for treatment of wet AMD. On the other hand, the immunocompromised patients with CMV retinitis often need long-term antiretroviral therapy (e.g. ganciclovir) to prevent vision loss[7]. To date, intravitreal and subretinal injection are the only two options of administrating therapeutic agents to the retina in clinical practice.
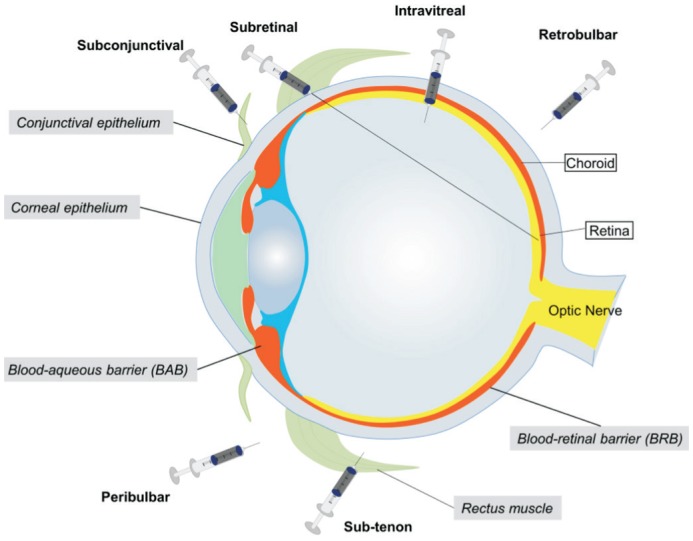
The primary reason of drug delivery limitation is the multiple physical boundaries (Figure 1) within the eye, which consist of corneal and conjunctival epithelium, blood-aqueous barriers (BAB), and blood-retinal barriers (BRB). These structures restrict the passage of molecules and fluids to the retina and also impede drug penetration[8]. Conventional drug administration systems such as eye drops, suspensions, and ointments are usually the most convenient methods to administer ophthalmic medications but they mainly target the anterior segment of the eye[9]. To treat retinal diseases, conventional dosage forms (i.e. eye drops), need to traverse the anterior chamber before the drug is able to penetrate deeply into the posterior tissues. The main barriers of the anterior chamber are the corneal and conjunctival epithelium. Besides those physical barriers, other anatomical and physiological processes, such as reflex blinking, tear turnover, and nasolacrimal drainage further lower ocular bioavailability of the drug. Therefore, topical instillation is rarely used in the treatment of retinal diseases because of the lack of exposure to the retina[10]. To enhance drug retention in the cornea and conjunctiva, pharmaceutical companies have developed topical gel systems that prolong drug exposure, particularly for dry eye syndrome[11]. These gels are polymer-based semisolid materials with high viscosity, such as hypromellose gel (GenTeal®, Alcon laboratories, Inc., Texas, USA) used for the relief of dry eye[12]. This formulation significantly reduces the dosing frequency compared with eye drops but is still not able to enhance the drug permeation to the retinal tissue. For systemic administration, the BRB that includes capillary endothelial cells (inner BRB) and retinal pigment epithelial cells (RPE cells, outer BRB) pose the most formidable obstacles for drug delivery due to the innate protective nature of the demarcation from the rest of the circulation[13]. Currently, numerous cell culture models of ocular barriers models are available as highly reproducible and convenient systems for nanoparticle permeability studies. The common models include primary cell culture and immortalized cell lines that represent the corneal, inner, and outer BRB separately. Some of them have been commercialized and can be purchased from major cell banks. Altogether, a challenging task in retinal drug delivery is to overcome these protective barriers of the eye and deliver the drug with minimal damage to the retinal tissues; a result, there has been a greater interest in research focused on the development of new nanotechnology-based drug delivery systems to facilitate retinal drug administration.
Figure 1. Schematic illustration of main ocular barriers and various routes of drug administration.
NANOTECHNOLOGY IN RETINAL DRUG DELIVERY
In the area of pharmaceutical sciences, nanomaterials are commonly interpreted as the application of nano-sized (1-1000 nm) tools in medicine, which mainly refers to all kinds of nanoparticles[14]. In contrast to atoms and macroscopic materials, nanomaterials have a high ratio of inner and outer surface area to volume, making them suitable candidates for carrying different drugs as well as attaching targeting moiety. Furthermore, different nanoparticles can be engineered to have a wide range of sizes, shapes, and surface chemical characteristics. By attaching an ophthalmic agent to nanoparticles, it is possible to improve the solubility of poorly water-soluble drugs, target the drug to the retina, enhance the cellular uptake of the drug, aid the transport of drug through biological barriers, increase the residence time, and protect the drug from degradation[14]. With these advantages, nanomedicine technology offers a great platform for designing a minimally or non-invasive system to deliver drugs to the retina in a sustained manner.
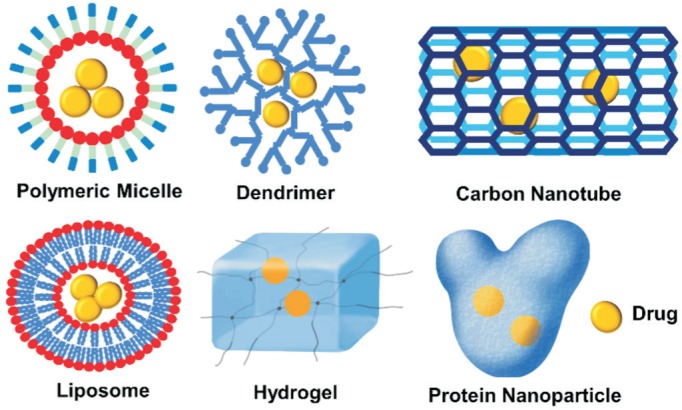
Remarkable advances in the field of drug delivery and material science in recent decades have led to the development of numerous nanomaterials. The most commonly used materials for nanoparticles include synthetic polymers (polymeric micelles, dendrimers, hydrogel), lipids (liposomes), proteins (albumin nanoparticles), and even inorganic compounds (Cerium oxide nanoparticles)[15]. Polymeric micelles are self-assembled from amphiphilic copolymers with a hydrophobic core and hydrophilic shell; dendrimers are highly branched tree-structured macromolecules; hydrogels are 3D networks of hydrophilic-polymers with high level of water content (up to 99% by weight); liposomes are self-assembling structures composed of lipid bilayers enclosing an aqueous core; albumin nanoparticles function by attaching drugs to the surface of the protein albumin, a natural protein abundant in the serum of most animals (Figure 2).
Figure 2. Representative examples of nanoparticles for retinal drug delivery.
Liposomes are small vesicles made of a lipid bilayer that can carry both hydrophilic and lipophilic drugs. Some of the benefits provided by liposome formulations are improvements in drug pharmacokinetics and pharmacodynamics, possibility for sustained release of medications, targeting of tissues, and a decrease in systemic toxicity[16]. Examples of chemotherapy drugs that have been approved in a liposomal form include doxorubicin, daunorubicin, and epirubicin. These drugs, when given in a liposomal form have less systemic side effects including cardiotoxicity, myelosuppression and nausea/vomiting among others[17]. In the case of ophthalmic drug delivery, the use of liposomes have potential to provide similar benefits as mentioned above. The effects of intravitreal liposomes encapsulating tacrolimus have been investigated for experimental autoimmune uveoretinitis (EAU) in Lewis rats. Tacrolimus remained in ocular fluids for 14d after intravitreal injection and it reduced intraocular inflammation and inhibited the development of EAU without any toxic effects[18]. Pentablock (PB) copolymer is another example of composite nanoformulation facilitating sustained delivery of biological therapeutics. A thermosensitive gelling copolymer encapsulating immunoglobulin-G fragment (IgG-Fab) as the mock drug was developed to achieve longer drug release. In vitro results demonstrated that the burst release was negligible for that formulation and a continuous near zero-order release sustained up to 80d. Those results are very encouraging but more studies have to be conducted to determine if the results from the in vitro studies can be translated to in vivo[19].
To utilize nanotechnology effectively for ocular drug delivery, special attention needs to be paid to nanoparticle size, shape, material and surface properties. The ideal size should be large enough to prevent their rapid leakage into the blood vessels but small enough to be injectable or to penetrate through the biological barriers toward the retina[20]. A study found that after intravitreal administration, larger particles (2 µm) were found to remain in the vitreous cavity, whereas the 200 nm particles were found evenly distributed in the vitreous cavity and the inner limiting membrane. The smaller 50 nm particles were detected in the retina even after 2mo post injection[21]. It should be noted that nanoparticles with similar sizes but different surface properties may express different distributions inside the eye[22], which provide valuable information for optimizing the physical-chemical properties of nanoparticles for retinal drug delivery. On the other hand, nanoparticles can be constructed based on needs of different drugs which can't be delivered directly by other routes. In addition, therapeutic agents have a higher chance of reaching specific areas at the back of the eye to treat sight-threatening retinal diseases. Clinical studies examining the efficacy and safety of such delivery systems are still in progress[23]. The ideal material for nanoparticles should be inert and biocompatible. A number of excipients approved by the FDA for human use should meet this need, such as poly lactic-co-glycolic acid (PLGA), poly (ethylene glycol) (PEG), and poly (hydroxyalkanoates) (PHAs). Moreover, bovine serum albumin (BSA), chitosan, Eudragit RS (ERS), and poly acrylic acid (PAA) have been used for ocular drug delivery[13]. In summary, a variety of nanostructures that differ in size, shape, structure, and material have been utilized for the administration of ophthalmic drugs.
Despite the advantages of nanoparticles, there is a growing concern about their potential toxicity, especially for those nanoformulations engineered with synthetic materials[24]. Currently, although most published nanoparticles claimed to be nontoxic or minimally toxic to cell lines or animals, very few studies have focused on identifying nanoparticle associated toxicity. It was reported that high doses of cyclodextrin siRNA nanoparticle by intravenous injection have led to multiple tissue toxicities[25]. However, It is still unclear the toxic response is attributed the nanomaterials. Nevertheless, the nanomaterial toxicity remained to be examined by comprehensive and long-term studies in future.
VARIOUS ADMINISTRATION ROUTES FOR RETINAL DRUG DELIVERY
Drug delivery strategies targeting the retina range from systemic administration to the clinically commonly used intravitreal injections. Representative examples of retinal drug delivery systems under research are listed in Table 1.
Table 1. Representative examples of retinal drug delivery system.
| Particle Type | Drug (Payload) | Application | Route | Year |
| PLGA | Bevacizumab | Wet AMD | Intravitreal | 2012[26] |
| Light-responsive | Nintedanib | Neovascularization | Intravitreal | 2015[27] |
| Pentablock copolymer | IgG-Fab fragment | General retinal diseases | Intravitreal | 2016[19] |
| PMMA dendrimer | Carboplatin | Retinoblastoma | Subconjunctival | 2014[28] |
| Liposome | Rpe65 DNA | Rpe65 associated blindness | Subretinal | 2014[29] |
| Naked siRNA | Claudin-5 siRNA | Modulation of BRB | Systemic | 2009[30] |
PLGA: Poly (lactic-co-glycolic acid); PMMA: Polymethylmethacrylate; RPE: Retinal pigment epithelium.
Intravitreal Route
Intravitreal injections were first reported in 1944 but not widely used until the 1990s[31], and continue to be the most common method for the treatment of retinal diseases, particularly for administration of macromolecules like bevacizumab (149 kDa). The 27 or 30-gauge needles are often used for injection of various drugs into the vitreous body. Under this route, the drug passively diffuses in all directions instead of directly targeting the retina following drug administration. Multiple nanomaterials have been utilized via the intravitreal route as discussed below.
The use of liposomes in ocular drug delivery has been explored by encapsulating bevacizumab to achieve sustained release of drugs via the dehydration-rehydration method[32]. After reducing the size of liposomes to the nanoscale with 0.22 µm filters, the final liposomal formulation was injected into the vitreous body of the rabbit models. The mean concentration of free bevacizumab in the eyes that received liposomal bevacizumab compared with the eyes that were injected with soluble bevacizumab was one and five times higher at day 28 and 42, respectively. Another formulation used to prolong the release of bevacizumab is PLGA nanoparticles. PLGA is an FDA-approved polymer with great biocompatibility and biodegradability[33]. It tends to degrade slowly in the body by hydrolysis of ester bonds between monomer units, leading to the release of the drug from the cavities of the particles. This property has been utilized to make a controlled-release formulation of bevacizumab[26],[34]. In contrast to liposomes, PLGA nanoparticles require low-cost materials and are more easily produced at large scales. Huu et al[27] used PLGA nanoparticles as the standard system to compare the efficacy with light-responsive nanoparticle. Both nanoparticles were used to deliver Nintedanib, a small molecule angiogenesis inhibitor.
Clinical studies have shown that intravitreal injections are typically safe procedures with a low risk for complications in most cases. However, the risks of complications should always be considered as physiological/disease conditions vary in the human populations. Some possible complications of intravitreal injections include ocular hypertension, inflammation, intraocular hemorrhage, infection within the eye (endophthalmitis), and rarely, retinal detachment. On the other hand, the estimated incidence of endophthalmitis associated with intravitreal injections of triamcinolone acetonide ranged from 0.86% to 6.73% per injection, much higher than the incidence with anti-VEGF compounds, which ranges from 0.019% to 0.077% per injection[35]. This adverse effect may be attributed to multiple factors, such as the use of preservatives, large size of needles and triamcinolone-induced immunosuppression. This unmet medical need points towards an important direction for the future work of nanotechnology research.
Periocular Route
The periocular route refers to the use of space surrounding the eyeball but within the orbit to deliver drugs. In contrast to intravitreal administration, the periocular route is a minimally invasive alternative for therapeutics targeting the retina because sclera is not be punctured, thereby greatly improving patient compliance and safety. Periocular pathways used for retinal drug delivery include four sub-routes: the subconjunctival, retrobulbar, peribulbar, and sub-tenon routes. The subconjunctival space is closest to the outside of the eyeball while the retrobulbar site is behind the eyeball. The sub-tenon and peribulbar spaces are between them, along with the orbit. The periocular route doesn't require the drug to penetrate through the corneal and conjunctival epithelium, but the sclera has to be traversed before the drug reaches the retina irrespective the sub-route used.
Currently, the subconjunctival injection of drug-loaded nanoparticles has been most commonly explored among various periocular routes. The subconjunctival space is just beneath the conjunctival membrane that covers the sclera and is easier to reach than other periocular spaces. It is reported that a biodegradable and thermosensitive gel, through subconjunctival administration, could provide sustained release of ovalbumin to the choroid and retina[36]. The ovalbumin concentrations were maintained at measurable levels in the sclera, choroid, and retina of rats over a period of 14d. This method provides an easier and safer way to target the retina, compared to other methods such as ocular insertion of solid implants. This study also indicated that the macromolecule ovalbumin proteins are able to traverse RPE cells to the retinal layer; however, this still needs more experimental verification.
Carboplatin is a chemotherapy drug used against retinoblastoma, an eye cancer often found in children, and subconjunctival administration of carboplatin loaded dendrimers to treat murine retinoblastoma has been explored. The results of the study showed that nanoparticles encapsulated with carboplatin were more effective than carboplatin in aqueous solution in terms of reducing tumor burden[37]. Another study reported highly efficient loading of hydrophilic carboplatin in a hydrophobic polymethylmethacrylate (PMMA) nanocarrier, a biocompatible and slowly biodegradable acrylic polymer, and compared its ocular kinetics to the bare carboplatin. This nanoparticle displayed a higher trans-scleral permeability gradient in the vitreous, leading to sustained higher levels of carboplatin for the treatment for advanced retinoblastoma[28].
Hydrogels are another type of material used to deliver drugs, including insulin, to treat DR via subconjunctival injection. One study showed that the insulin released from the hydrogels could be detected in the retina by using confocal microscopy and significantly reduced the DNA fragmentation of diabetic retinas one week post subconjunctival implantation[38]. In addition, hydrogels can be engineered to possess remarkable biocompatibility, hydrophilicity, flexibility, and elasticity making them highly suitable for ocular drug delivery and targeting[39]. More sophisticated stimuli-responsive hydrogel systems are also emerging, such as ion-, pH-, and thermo-sensitive hydrogels that undergo reversible volume or sol-gel phase transition upon certain stimuli[40].
Collectively, the periocular route can be used as an alternative choice for ocular drug delivery because it deposits agents adjacent to the target tissues, such as the choroid and retina, and is less invasive than the intravitreal route, however, some anterior segment complications such as intraocular pressure, hyphema, and cataracts have been observed following periocular injections[41].
Subretinal Route
The subretinal (under the retina) route is another site explored for retinal drug delivery. The subretinal cavity is the space between RPE cells and photoreceptors, and the injected materials come into direct contact with the targeted RPE cells through this route[42]. The subretinal injection is typically more invasive than other routes of injection but relatively higher concentrations of therapeutic agents can be reached due to minimal dilution within the cavity[43]. This makes it an excellent site for drug delivery, especially for transfection of RPE cells in gene delivery.
In 2014, the subretinal route was used to inject a non-viral biocompatible, liposome-protamine-DNA complex to deliver retinal pigment epithelium 65 (RPE65) gene, a critical gene responsible for the regeneration of the visual pigment, to the retina. Long-term expression of RPE65 gene in knockout mice, that lacked the native RPE65 gene, led to in vivo correction of blindness[29]. On Dec. 2017, voretigene neparvovec-rzyl (Luxturna®; Spark Therapeutics, Inc., PA, USA), a virus vector-based gene therapy administered by subretinal injection, was approved by FDA to treat patients with RPE65 mutation-associated retinal dystrophy[44]. Novel nanotechnology currently in lab research may be translated to future voretigene neparvovec-rzyl formulation.
Systemic Route
For decades, the systemic administration has been considered infeasible in ocular drug delivery because less than 2% of systematically administered drugs could reach the vitreous[45]. As noted above, the passage of a drug is prohibited by the blood-retinal barrier, especially in the case of drug delivery to the posterior segment of the eye where the direction of drug penetration is opposite to the direction of intraocular liquid circulation[10]. Despite lower efficacy, the systemic route still presents an attractive strategy since it doesn't involve penetration into ocular tissues, making it very convenient especially for outpatients.
Miettinen et al[46] explored a novel strategy to overcome the ocular tissue obstacle by successfully opening the BRB reversibly and transiently with RNAi technology[30]. The RNAi target was the claudin-5 protein, a critical component of tight junctions. Upon injection of claudin-5 siRNA into the mice tail vein, claudin-5 was down-regulated in the retina and permeability of microvessels was increased 48h post-administration. After 72h, the level of permeability returned to normal levels. The limitation of this study is the specificity of claudin-5. Claudin-5 is expressed in many other tissues such as glandular and ductal epithelia. Further investigation into this research is needed to verify the adverse effects at the center of concern. Aside from pharmacological intervention, there is evidence that BRB breakdown is naturally induced in some situations, such as ocular traumas and diabetes[47]. The understanding of the pathophysiological mechanisms of the BRB is still evolving with new research, and these findings have the potential to provide new possibilities for the application of systemic administration in retinal drug delivery.
CONCLUSION AND FUTURE PERSPECTIVES
Nanotechnology has evolved considerably since its advent and is undergoing different developmental stages. Intravitreal, periocular, subretinal, and systemic drug delivery systems all have their advantages and drawbacks. The nanotechnology-based delivery systems used in those routes offer promising alternatives for overcoming the limitations of the frequent and long-term intravitreal injection. Minimally invasive therapy with prolonged efficacy remains a major unmet medical need for patients with retinal diseases. In the intravitreal route, the frequency of injections needs to be optimized without compromising the efficacy and duration of pharmacological activity for intended therapeutic agents. The research of systemic drug delivery is in a very early stage, where determining how to avoid systemic toxicity while delivering adequate drug across the BRB is a challenging goal. The periocular route offers the most promising alternative for enhanced drug delivery with a reduced risk of ocular complications and systemic toxicity but there are some other limitations, such as inadequate tissue distribution.
In recent years, therapeutic biologics have become the mainstay of anti-VEGF treatment (e.g. aflibercept, ranibizumab, and bevacizumab)[48]. Therefore, drug delivery systems tailored for biologics have gained tremendous interest for both academia and industry. One of the promising systems is injectable and sustained-release hydrogels for localized drug delivery[49]. An ongoing study is industry-funded and holds a great commercialization potential of leading to a better treatment for wet AMD, retinal vein occlusion (RVO) and other retinal diseases. This formulation is expected to optimize aflibercept therapy, reducing dosing frequency and subsequent doctor visits, thus decreasing the medical burden for both patients and health care providers[50]. We believe that more and more drug delivery systems for biologics will be engineered and subsequently be marketed to benefit the patients in future.
Overall, based on our literature search it is evident that nanotechnology has extensive advantages in retinal drug delivery such as enhanced drug solubility and bioavailability, improved drug penetration, larger absorption area, and less frequent injections[51]. Despite those benefits, safety always remains a concern to bring new drugs or new formulations from bench to bedside. A few other questions also remained to be addressed, such as: which nanoparticle is the most suitable for clinical use? how can nanoparticles production be scaled up with minimal cost of manufacturing? In addition to the aforementioned nanotechnology, stem cell therapy, ocular implants, and needle devices are also being explored for the treatment of retinal diseases. Respective reviews of these systems have been published recently[7],[10],[52]. With interdisciplinary collaboration across the fields of science, engineering, and medicine, better ophthalmic treatments are expected to become available for clinical use in near future. These advancements hold great promise for “smart” drug delivery systems that will make the available treatment for retinal diseases much safer, more convenient, and more effective.
Acknowledgments
The authors would like to thank Tabassum Shafi for assistance of literature review.
Conflicts of Interest: Jiang S, None; Franco YL, None; Zhou Y, None; Chen J, None.
REFERENCES
- 1.Dilnawaz F, Sahoo SK. Nanotechnology-Based Ophthalmic Drug Delivery System. In: Domb JA, Khan W, editors. Focal Controlled Drug Delivery. Book; 2014. pp. 225–241. [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed in May, 2018]. Available at: https://www.cdc.gov/visionhealth/basics/ced/index.html.
- 3.Bisht R, Mandal A, Jaiswal JK, Rupenthal ID. Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2017;10(2):e1473. doi: 10.1002/wnan.1473. [DOI] [PubMed] [Google Scholar]
- 4.Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine (Lond) 2017;12(6):683–702. doi: 10.2217/nnm-2016-0379. [DOI] [PubMed] [Google Scholar]
- 5.Zafir-Lavie I, Michaeli Y, Reiter Y. Novel antibodies as anticancer agents. Oncogene. 2007;26(25):3714–3733. doi: 10.1038/sj.onc.1210372. [DOI] [PubMed] [Google Scholar]
- 6.Yonekawa Y, Kim IK. Clinical characteristics and current treatment of age-related macular degeneration. Cold Spring Harb Perspect Med. 2014;5(1):a017178. doi: 10.1101/cshperspect.a017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vadlapudi AD, Vadlapatla RK, Mitra AK. Current and emerging antivirals for the treatment of cytomegalovirus (CMV) retinitis: an update on recent patents. Recent Pat Antiinfect Drug Discov. 2012;7(1):8–18. doi: 10.2174/157489112799829765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13(3-4):135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kim YC, Chiang B, Wu X, Prausnitz MR. Ocular delivery of macromolecules. J Control Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Kambhampati SP, Kannan RM. Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol. 2013;20(1):26–37. doi: 10.4103/0974-9233.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye(Lond) 2011;25(5):578–586. doi: 10.1038/eye.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauber J. Efficacy, tolerability and comfort of a 0.3% hypromellose gel ophthalmic lubricant in the treatment of patients with moderate to severe dry eye syndrome. Curr Med Res Opin. 2007;23(11):2629–2636. doi: 10.1185/030079907x233197. [DOI] [PubMed] [Google Scholar]
- 13.Nakhlband A, Barar J. Impacts of nanomedicines in ocular pharmacotherapy. Bioimpacts. 2011;1(1):7–22. doi: 10.5681/bi.2011.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8(6):2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 15.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine(Lond) 2013;8(9):1509–1528. doi: 10.2217/nnm.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, He R, Qian JA, Guo J, Xue K, Yuan YF. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of tacrolimus (FK506) encapsulated in liposomes. Invest Ophthalmol Vis Sci. 2010;51(7):3575–3582. doi: 10.1167/iovs.09-4373. [DOI] [PubMed] [Google Scholar]
- 19.Agrahari V, Agrahari V, Hung WT, Christenson LK, Mitra AK. Composite nanoformulation therapeutics for long-term ocular delivery of macromolecules. Mol Pharm. 2016;13(9):2912–2922. doi: 10.1021/acs.molpharmaceut.5b00828. [DOI] [PubMed] [Google Scholar]
- 20.Kim TW, Lindsey JD, Aihara M, Anthony TL, Weinreb RN. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci. 2002;43(6):1809–1816. [PubMed] [Google Scholar]
- 21.Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33(1):31–36. doi: 10.1159/000055638. [DOI] [PubMed] [Google Scholar]
- 22.Koo H, Moon H, Han H, Na JH, Huh MS, Park JH, Woo SJ, Park KH, Kwon IC, Kim K, Kim H. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials. 2012;33(12):3485–3493. doi: 10.1016/j.biomaterials.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.El-Ansary A, Al-Daihan S, Bacha AB, Kotb M. Toxicity of novel nanosized formulations used in medicine. Methods Mol Biol. 2013;1028:47–74. doi: 10.1007/978-1-62703-475-3_4. [DOI] [PubMed] [Google Scholar]
- 25.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, Kornbrust DJ, Davis ME. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104(14):5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Hurley B, Liu Y, Leonard B, Griffith M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol J. 2012;6:54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huu VA, Luo J, Zhu J, Zhu J, Patel S, Boone A, Mahmoud E, McFearin C, Olejniczak J, de Gracia Lux C, Lux J, Fomina N, Huynh M, Zhang K, Almutairi A. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J Control Release. 2015;200:71–77. doi: 10.1016/j.jconrel.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shome D, Kalita D, Jain V, Sarin R, Maru GB, Bellare JR. Carboplatin loaded polymethylmethacrylate nano-particles in an adjunctive role in retinoblastoma: an animal trial. Indian J Ophthalmol. 2014;62(5):585–589. doi: 10.4103/0301-4738.129792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajala A, Wang Y, Zhu Y, Ranjo-Bishop M, Ma JX, Mao C, Rajala RV. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014;14(9):5257–5263. doi: 10.1021/nl502275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell M, Nguyen AT, Kiang AS, Tam LC, Gobbo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, Humphries P. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci U S A. 2009;106(42):17817–17822. doi: 10.1073/pnas.0908561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peyman GA, Vastine DW, Meisels HI. The experimental and clinical use of intravitreal antibiotics to treat bacterial and fungal endophthalmitis. Doc Ophthal. 1975;39(1):183–201. doi: 10.1007/BF00578762. [DOI] [PubMed] [Google Scholar]
- 32.Abrishami M, Zarei-Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina(Philadelohia, Pa) 2009;29(5):699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Banderas L, Duran-Lobato M, Munoz-Rubio I, Alvarez-Fuentes J, Fernandez-Arevalo M, Holgado MA. Functional PLGA NPs for oral drug delivery: recent strategies and developments. Mini Rev Med Chem. 2013;13(1):58–69. [PubMed] [Google Scholar]
- 34.Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomedicine. 2009;4:175–183. doi: 10.2147/ijn.s6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. BrJ Ophthalmol. 2013;97(4):460–465. doi: 10.1136/bjophthalmol-2012-302435. [DOI] [PubMed] [Google Scholar]
- 36.Rieke ER, Amaral J, Becerra SP, Lutz RJ. Sustained subconjunctival protein delivery using a thermosetting gel delivery system. J Ocu Pharmacol Ther. 2010;26(1):55–64. doi: 10.1089/jop.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SJ, Durairaj C, Kompella UB, O'Brien JM, Grossniklaus HE. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol. 2009;127(8):1043–1047. doi: 10.1001/archophthalmol.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imai H, Misra GP, Wu L, Janagam DR, Gardner TW, Lowe TL. Subconjunctivally implanted hydrogels for sustained insulin release to reduce retinal cell apoptosis in diabetic rats. Invest Ophthalmol Vis Sci. 2015;56(13):7839–7846. doi: 10.1167/iovs.15-16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fathi M, Barar J, Aghanejad A, Omidi Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts. 2015;5(4):159–164. doi: 10.15171/bi.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kushwaha SK, Saxena P, Rai A. Stimuli sensitive hydrogels for ophthalmic drug delivery: a review. Int J Pharm Investig. 2012;2(2):54–60. doi: 10.4103/2230-973X.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misra GP, Singh RS, Aleman TS, Jacobson SG, Gardner TW, Lowe TL. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials. 2009;30(33):6541–6547. doi: 10.1016/j.biomaterials.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao Y, Wang Y, Ma Z, Wang L, Qin L, Wang L, Huang YF, Zhang S. Subretinal delivery of erythropoietin alleviates the N-methyl-N-nitrosourea-induced photoreceptor degeneration and visual functional impairments: an in vivo and ex vivo study. Drug Deliv. 2017;24(1):1273–1283. doi: 10.1080/10717544.2017.1370620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nickerson JM, Goodman P, Chrenek MA, Bernal CJ, Berglin L, Redmond TM, Boatright JH. Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. Methods Mol Biol. 2012;884:53–69. doi: 10.1007/978-1-61779-848-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. [Accessed in May, 2018]. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm.
- 45.Hosoya K, Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharm Res. 2009;26(9):2055–2065. doi: 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- 46.Miettinen M, Sarlomo-Rikala M, Wang ZF. Claudin-5 as an immunohistochemical marker for angiosarcoma and hemangioendotheliomas. Am J Surg Pathol. 2011;35(12):1848–1856. doi: 10.1097/PAS.0b013e318229a401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Occhiutto ML, Freitas FR, Maranhao RC, Costa VP. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics. 2012;4(2):252–275. doi: 10.3390/pharmaceutics4020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliano R. Challenges to macromolecular drug delivery. Biochem Soc Trans. 2007;35(Pt 1):41–43. doi: 10.1042/BST0350041. [DOI] [PubMed] [Google Scholar]
- 49.Wang K, Han Z. Injectable hydrogels for ophthalmic applications. J Control Release. 2017;268:212–224. doi: 10.1016/j.jconrel.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams B. Regeneron pumps $300M-plus into Ocular Therapeutix for new Eylea formulation. [Accessed in May, 2018]. Available at: https://www.fiercebiotech.com/biotech/regeneron-pumps-300m-plus-into-ocular-therapeutix-for-new-eylea-formulation.
- 51.Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188. doi: 10.3389/fphar.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer N, Narayanan R, Loewenstein A, Kuppermann BD. Drug delivery to the posterior segment of the eye. Eur J Ophthalmol. 2011;21(Suppl 6):S20–S26. doi: 10.5301/EJO.2010.6051. [DOI] [PubMed] [Google Scholar]


