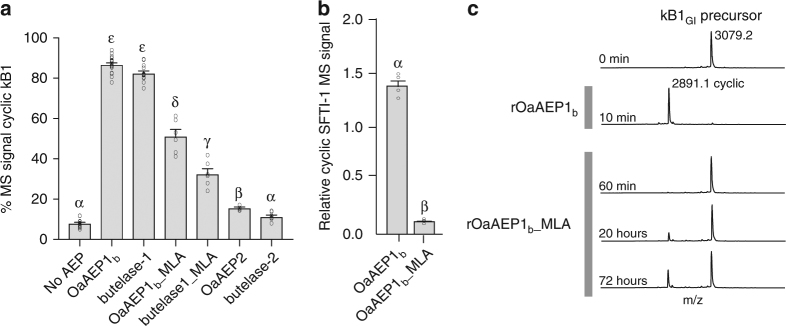
Fig. 4.
The MLA influences the yield of cyclic peptide in planta. a MALDI-MS analysis of N. benthamiana-produced peptides upon co-expression of AEP variants with Oak1. For all treatments, means and s.e.m. are of the percentage MS signal obtained for cyclic kB1 over the total MS signal of all kB1-related peptides produced. Treatments carrying unique Greek lettering are significantly different (P < 0.05) as determined by Tukey’s ANOVA. Replicates are as follows: n = 14 (No AEP), n = 16 (OaAEP1b), n = 13 (butelase1), n = 9 (OaAEP1b_MLA), n = 6 (butelase1_MLA), n = 6 (OaAEP2), and n = 8 (butelase2). Error bars are s.e.m. b Relative abundance of cyclic SFTI-1 in leaf extracts after co-expression of Oak1_SFTI-1 (Supplementary Fig. 6) with OaAEP1b (n = 3) or OaAEP1b_MLA (n = 3). Error bars are s.e.m. Treatments carrying unique Greek lettering are significantly different (P < 0.05) as determined by Tukey’s ANOVA. c In vitro comparison of the enzyme activity preference (protease vs ligase) of recombinant OaAEP1b and OaAEP1b_MLA. Representative MALDI-MS profiles (n = 3) of the kB1 precursor (kB1GI) incubated with recombinant OaAEP1b and OaAEP1b_MLA (each at 23.5 µg mL−1 total protein) for up to 72 h. For rOaAEP1b, all precursor peptides were converted to cyclic kB1 (2891.1 m/z) within 10 min, while substantial precursor peptide remained in the case of OaAEP1b_MLA even after 72 h of incubation. No linear kB1 peptide was detected from either enzyme. Observed monoisotopic masses (Da; [M + H]+) are listed