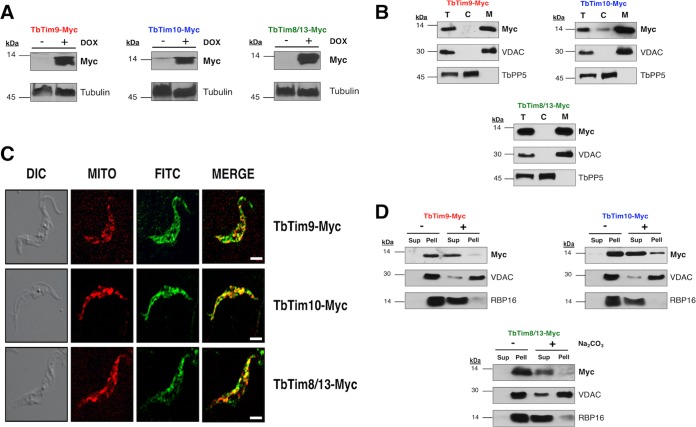
FIG 2 .
Expression and mitochondrial localization of the small TbTim proteins. (A) Inducible expression of TbTim9-Myc, TbTim10-Myc, and TbTim8/13-Myc in T. brucei. Cells were grown in the absence (−) and presence (+) of doxycycline (DOX) for 48 h, and total cellular proteins were analyzed by SDS-PAGE and immunoblot analysis using anti-myc monoclonal antibody. Tubulin was used as a loading control. (B) Subcellular fraction of T. brucei cells expressing TbTim9-Myc, TbTim10-Myc, or TbTim8/13-Myc. Proteins from the total cell lysates (lanes T), cytosolic fractions (lanes C), and mitochondrial fractions (lanes M) were subjected to SDS-PAGE and were probed with anti-myc antibody. VDAC and TbPP5 were used as the mitochondrial and cytosolic protein markers, respectively. (C) In situ immunofluorescence staining of TbTim9-Myc, TbTim10-Myc, and TbTim8/13-Myc cells. Live cells were stained with MitoTracker Red and then stained with anti-myc antibody as the primary antibody and FITC-conjugated anti-mouse IgG as the secondary antibody. Confocal microscopy images were taken using an LSM510 confocal microscope at ×60 magnification. The differential interference contrast (DIC), MitoTracker Red (MITO), and FITC images were merged to show colocalization. Size bar, 5 µm. (D) Alkaline sodium carbonate extraction of mitochondria isolated from T. brucei cells expressing the ectopic small TbTim proteins. Mitochondria were treated (+) with 100 mM sodium carbonate (pH 11.5) or left untreated (−) on ice for 30 min. Soluble (Sup) and insoluble (Pell) fractions were separated by centrifugation, and proteins were analyzed by Western blotting using anti-myc antibodies. VDAC and RBP16 were used as markers for membrane-bound and soluble proteins, respectively.