Figure 1.
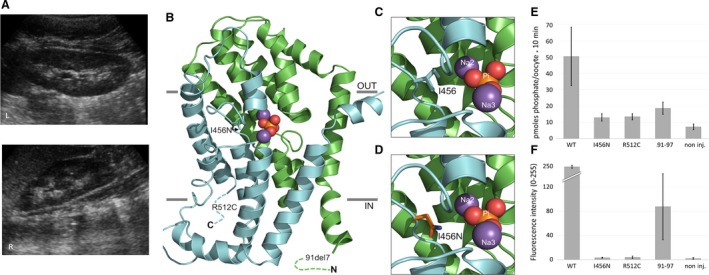
Clinical, structural, and functional characterization of SLC34A1 mutations. (A) Medullary nephrocalcinosis seen in patient B. Renal ultrasound images of left and right kidneys are shown, demonstrating bilateral nephrocalcinosis. (B, C, D) Homology model of NaPi‐IIa (residues 97–502) with the two structural repeats (RU1 and RU2) highlighted in green and cyan, respectively. Bound phosphate (Pi) is shown as orange and red spheres and sodium ions as magenta spheres. Note that only approximate positions of the 91del7 and R512C mutants are indicated. The hydrophobic isoleucine at position 456 (I456) in RU2 of wild‐type NaPi‐IIa (C) is replaced in (D) by the polar asparagine residue (I456N). (E) Xenopus oocyte transport activity of wild‐type (WT) and mutated SLC34A1 ‐ GFP‐coupled constructs. Transport activity was determined by [32P]phosphate flux measurements. WT significantly stimulates [32P]phosphate uptake compared with non‐injected oocytes (P < 0.01, ANOVA post hoc Tukey). The slightly increased transport rate with the mutants is not significant. (B) Surface expression of SLC34A1‐GFP constructs in Xenopus oocytes assessed by fluorescence microscopy. Fluorescence was quantified using image J, the average intensity of eight oocytes is shown (range 0–255 AU).
