Figure 6.
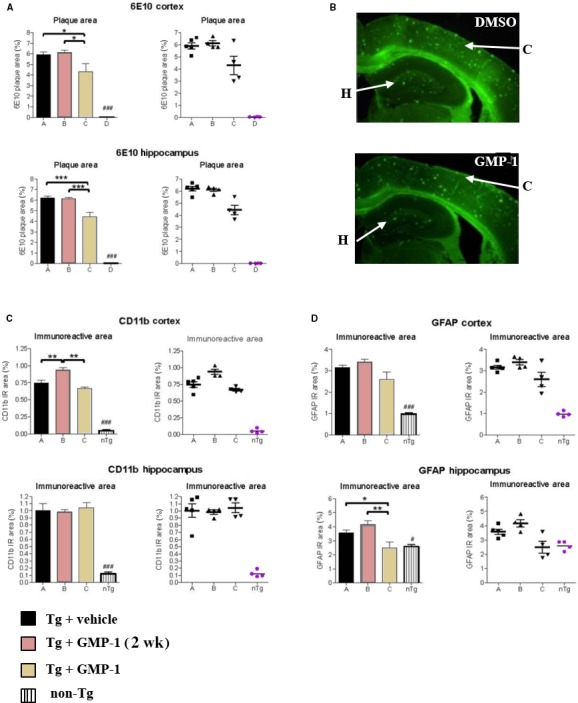
Immunohistochemical assessment of GMP‐1 treatment on amyloid plaques accumulation and neuroinflammation. A, Amyloid plaque area in cortex and hippocampus measured as anti‐APP (6E10) antibody staining. Placebo‐treated, GMP‐1 chronically treated, GMP‐1 acutely treated 5xFAD mice as well as non‐transgenic animals indicated as A, B, C and D, respectively. Differences between groups were calculated by one‐way ANOVA followed by a Newman‐Keuls post‐hoc test, the alpha‐error set to 0.05. *P < .05, ***P < .001, n = 6. B, Representative images of brain slices from 6‐month‐old 5xFAD mice stained with 6E10 antibodies and Alexa Fluor® 488 secondary antibodies. Arrows indicate area of cortex (C) and hippocampus (H). C, Astrocytosis in the cortex and hippocampus measured as anti‐GFAP antibody staining. Placebo‐treated, GMP‐1 chronically treated, GMP‐1 acutely treated 5xFAD mice as well as non‐transgenic animals indicated as A, B, C and D, respectively. Differences between groups were calculated by one‐way ANOVA followed by a Newman‐Keuls post‐hoc test, the alpha‐error set to 0.05. *P < .05, **P < .01, n = 6. D. Microglia activation in the cortex and hippocampus measured as anti‐CD11b antibody staining. Placebo‐treated, GMP‐1 chronically treated, GMP‐1 acutely treated 5xFAD mice as well as non‐transgenic animals indicated as A, B, C and D, respectively. Differences between groups were calculated by one‐way ANOVA followed by a Newman‐Keuls post‐hoc test, the alpha‐error set to 0.05. **P < .01, n = 6
