Figure 1.
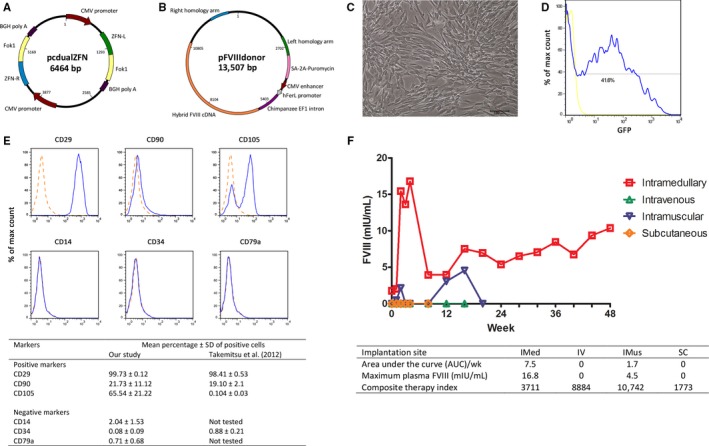
Maps of plasmids for ZFN‐mediated integration of donor FVIII transgene, immunophenotype of primary canine BMSC, and time course of plasma transgenic FVIII protein levels after autologous BMSC implantation in intramedullary and extramedullary sites. A, pcdualZFN expressed ZFN from dual expression cassettes of FokI endonuclease monomers and zinc finger peptides (ZFN‐L and ZFN‐R) targeted at canine PPP1R12C intron 1 (Sigma‐Aldrich, St. Louis, MO, USA). FokI monomers were modified for obligate heterodimerization and enhanced nuclease activity as previously described.9 B, pFVIIIdonor provided cDNA encoding a hybrid human‐porcine B domain‐truncated FVIII (5.1 kb) expressed from the human ferritin light chain promoter 10 flanked by 0.8 kb homology sequences at the ZFN target site. C, Primary canine BMSCs expanded ex vivo. Scale bar = 100 μm. D, Flow cytometry profile of GFP‐positive primary BMSCs of dog #5954 (intramedullary implantation) 24 h after electroporation with a GFP‐expressing plasmid. E, A representative profile of positive and negative markers of mesenchymal stromal cells present in early passage BMSCs in this study analysed by flow cytometry as previously described.4 Broken lines denote the corresponding isotype control. Bone marrow was obtained by aspiration through the trochanteric fossa of the femur. Summary of mesenchymal stromal cell markers of BMSCs of all dogs in our study compared with data of Takemitsu et al of primary bone marrow‐derived mesenchymal stem cells of 4 beagle dogs.13 The histogram subtraction method was used to calculate the mean percentage ± standard deviation of cells positive for each surface protein.15 All antibodies used for immunophenotyping were reactive against the cognate canine protein except anti‐CD105 antibody whose reactivity against canine CD105 was not specified by the manufacturer. F, Plasma levels of human FVIII protein after implantation of autologous ZFN‐treated BMSCs by the intramedullary method (IMed), intravenous infusion (IV), intramuscular (IMus) and Matrigel™‐encapsulated subcutaneous injection (SC). Summary of plasma human FVIII protein levels by implantation site and composite therapy index of each dog
