Figure 1.
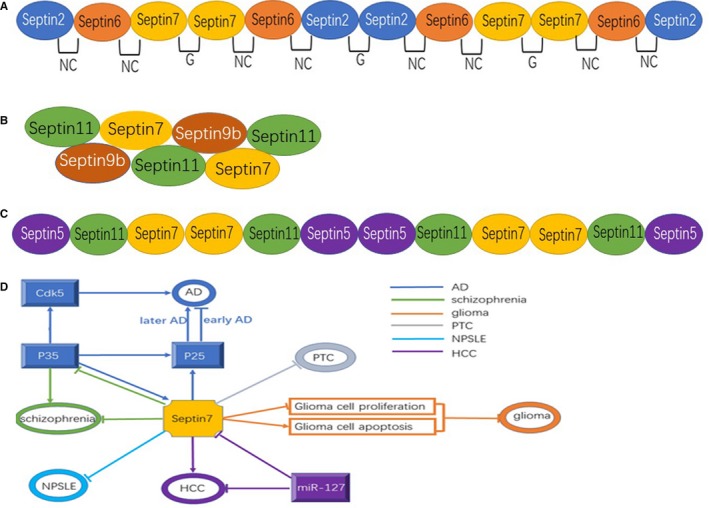
A, Organization chart of the septin2/6/7 complex. Septin 7 is a core component of septin 2/6/7. Septin 2/6/7 is the most abundant septin complex and the structure of the complex shows a universal bipolar polymer, composed of an extended G domain and/or the N‐ and C‐terminal extensions.24 Kinoshita et al identified that septin 2/6/7 is a non‐polar hexamer and 2 copies of each septin symmetrically arranged (septin 7/septin 6/septin 2/septin 2/septin 6/septin 7). B, Organization chart of the septin7/9b/11 complex. Septin 9b binds to C termini of both septin 7 and septin 11 through its long N‐terminal extension and septin 7/9b/11 forms a filamentous pattern along actin stress fibres in the actin filament‐dependent manner. Structure of the filaments containing septins 7/9b/11 depends on the integrity of actin filaments in REF52 cells. C, Organization chart of the septin5/7/11 complex. Septin 5 and septin 11 can colocalize and coimmunoprecipitate with septin 7. The existence of septin 5/7/11 complexes does not contradict the existence of previously reported septin 2/6/7 or septin 7/9b/11 complexes. D, Schematic Organization chart of the role of septin 7 in different diseases and the related regulation mechanism. The common structure of the septin 7 in human and other organisms and its molecular mechanism of action in physiology and disease pathology was summarized in various diseases including Alzheimer's disease (AD), schizophrenia, neuropsychiatric systemic lupus erythematosus (NPSLE), glioma, papillary thyroid carcinoma (PTC) and hepatocellular carcinoma (HCC)
