Abstract
Background/Objectives
This meta-analysis is aimed at investigating the prognostic roles of the inflammatory markers neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in patients with pancreatic cancer.
Methods
The correlations between high inflammatory marker expression levels and prognosis in 7105 patients with pancreatic cancer from 34 eligible studies were investigated. Additionally, subgroup analyses based on study location, tumor stage, treatment, and value cutoffs were performed.
Results
High NLR and PLR values were considered to be 2.0–5.0 and 150–200, respectively. Using a random-effects model, the estimated rates of high NLR and PLR were 0.379 (95% confidence interval [CI] 0.310–0.454) and 0.490 (95% CI 0.438–0.543), respectively. High NLRs were frequently found in patients with lower tumor stages and in those who underwent surgery. There were significant correlations between high NLR and PLR and poor survival rates (hazard ratio [HR] 1.737, 95% CI 1.502–2.009 and HR 1.143, 95% CI 1.037–1.259, resp.). Interestingly, the NLR and PLR had no prognostic value in patients who underwent chemoradiotherapy.
Conclusion
Taken together, our results showed that inflammatory markers are useful for predicting prognosis in patients with pancreatic cancer. The NLR is a more suitable parameter for predicting prognosis regardless of the patient's condition.
1. Introduction
Pancreatic cancer (PC) is one of the most lethal malignant neoplasms in the world [1]. The long-term prognosis of patients with PC is poor, with a median survival of 8.5–11 months for those with metastatic disease even with aggressive treatment [2, 3]. To date, surgical excision is the only curative treatment for PC; however, only 10–15% of patients are eligible for this procedure [4]. Chemo/radiotherapy is used to palliate symptoms and improve survival in patients with advanced disease. Therefore, accurate clinical staging and identification of prognostic factors are crucial for estimating prognosis and selecting appropriate treatment modalities.
Although the pathological stage of PC is considered the most significant prognostic factor, it is difficult to obtain tumor tissues for analysis in a significant number of patients. Recently, several studies have demonstrated that the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) could serve as a simple immune function index and may be of prognostic significance in patients with various solid tumors [5, 6]. However, the relationship between NLR/PLR and clinical outcomes in patients with PC remains controversial. As they are derived from routine laboratory tests, NLR and PLR are easy to obtain and may serve an important function in monitoring PC progression as well as in predicting patient survival. Therefore, we performed this study to evaluate the prognostic values of NLR and PLR in patients with PC.
2. Methods
2.1. Published Studies' Search and Selection Criteria
We followed the methods of Jeong et al. [7]. Relevant articles were obtained by searching the PubMed and MEDLINE databases through December 31, 2017. These databases were searched using the following key words: “pancreatic cancer,” “survival,” and “neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio.” The titles and abstracts of all searched articles were screened. Review articles were also screened to identify additional eligible studies. Articles related to studies of human PC (other than pancreatic neuroendocrine tumors) and those with data pertaining to the correlation between inflammatory markers and survival were included. Articles were excluded if they were case reports or nonoriginal articles, or if not written in English. This protocol was reviewed and approved by the Institutional Review Board of Eulji Hospital (approval number NON2017-002).
2.2. Data Extraction
Data from all eligible studies were extracted by two independent authors. The following data were extracted from each of the eligible studies [8–41]: the first author's name, year of publication, study location, number of patients analyzed, tumor stage, treatment modality, criteria for each inflammatory marker, rate of patients with high inflammatory marker values, and information on the correlations between inflammatory markers and survival. For quantitative aggregation of survival results, the correlations between inflammatory markers and the overall survival (OS) rates were analyzed according to the reported hazard ratio (HR) using one of three methods. In studies not quoting the HR or its confidence interval (CI), these variables were calculated from the presented data using the HR point estimate, log-rank statistic or its P value, and the O-E statistic (i.e., the difference between the numbers of observed and expected events) or its variance. If these data were unavailable, the HR was estimated using the total number of events, number of patients-at-risk in each group, and the log-rank statistic or its P value. Finally, if the only useful data were in the form of graphical representations of survival distributions, survival rates were extracted at specified times to reconstruct the HR estimate and its variance under the assumption that patients were censored at a constant rate during the time intervals [42]. The published survival curves were read independently by two authors to reduce variability. The HRs were then combined into an overall value using Peto's method [43].
2.3. Statistical Analyses
To perform the meta-analysis, all data were analyzed using the Comprehensive Meta-Analysis software package (Biostat, Englewood, NJ, USA). We investigated high values of inflammatory markers (the NLR and PLR) and their correlations with OS rates in patients with PC. Heterogeneity between the studies was checked by the Q and I2 statistics and expressed as P values. Additionally, sensitivity analysis was conducted to assess the heterogeneity of eligible studies and the impact of each study on the combined effect. Because eligible studies were evaluated in various populations with different tumor stages and treatments, a random-effects model was applied as it was more suitable than a fixed-effects model for interpreting the influence of inflammatory markers. Begg's funnel plot and Egger's test were used to assess publication bias; if significant publication bias was found, fail-safe N and trim-fill tests were performed to determine the degree of such bias. The results were considered statistically significant at P < 0.05.
3. Results
3.1. Selection and Characteristics of the Studies
One hundred forty-seven reports were retrieved from the database; 37 articles were excluded as they were duplicates while a further 36 were excluded because of insufficient or no information. Additionally, 40 reports were excluded because they described other diseases (n = 29), were published in a language other than English (n = 1), or were nonoriginal research articles (n = 10). Finally, 34 studies that encompassed 7105 patients with PC were included in this meta-analysis (Figure 1 and Table 1).
Figure 1.
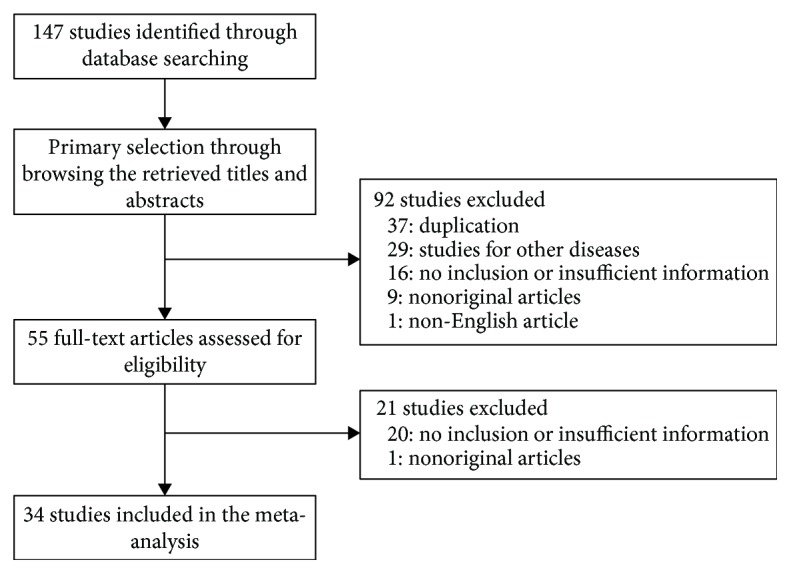
Flow diagram of the study selection process.
Table 1.
Main characteristics of the studies included in this meta-analysis.
| Author, year | Location | Tumor type | Tumor stage | Tx option | Parameter | Criteria | Criterion subgroup | Number of patients | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | High | Low | ||||||||
| Alagappan et al., 2016 [24] | USA | PDAC | ND | Mixed | NLR | 5 | High | 208 | ND | ND |
| PLR | 200 | High | 208 | ND | ND | |||||
| An et al., 2010 [36] | China | PC | III-IV | CTx | NLR | 5 | High | 95 | ND | ND |
| Asaoka et al., 2016 [21] | Japan | PC | I-III | Mixed | NLR | 2.7 | Low | 46 | 20 | 26 |
| Asari et al., 2016 [22] | Japan | PDAC | I-IV | Mixed | NLR | 3 | Low | 184 | 62 | 122 |
| Ben et al., 2015 [28] | China | PDAC | I-III | Surgery | NLR | 2 | Low | 381 | 267 | 114 |
| Chawla et al., 2017 [37] | USA | PDAC | I-IV | Mixed | NLR | 3.3 | Low | 217 | 107 | 110 |
| PLR | 175 | High | 217 | 107 | 110 | |||||
| Chen et al., 2017 [10] | China | PDAC | III-IV | CTx | NLR | 2.78 | Low | 132 | 78 | 54 |
| Cheng et al., 2016 [25] | China | PC | I-II | Mixed | NLR | 2 | Low | 195 | 128 | 67 |
| Inoue et al., 2015 [30] | Japan | PDAC | I-IV | Mixed | NLR | 2 | Low | 440 | 300 | 140 |
| PLR | 150 | Low | 440 | 201 | 239 | |||||
| Kishi et al., 2015 [27] | Japan | PC | III/IV | CTx/RTx | NLR | 5 | High | 65 | 7 | 58 |
| PLR | 150 | Low | 65 | 30 | 35 | |||||
| Kou et al., 2016 [23] | Japan | PC | III-IV | CTx | NLR | 5 | High | 306 | 49 | 257 |
| PLR | 150 | Low | 306 | 180 | 126 | |||||
| Lee et al., 2016 [19] | Korea | PDAC | III-IV | CTx | NLR | 5 | High | 82 | 20 | 62 |
| PLR | 150 | Low | 82 | 36 | 46 | |||||
| Liu et al., 2017 [14] | China | PDAC | I-IV | Mixed | NLR | 4.5 | High | 386 | 63 | 323 |
| Luo et al., 2015 [29] | China | PDAC | III-IV | CTx | NLR | 3.1 | Low | 403 | 194 | 209 |
| Martin et al., 2014 [31] | Australia | PC | III-IV | CTx/RTx | NLR | 5 | High | 124 | 60 | 64 |
| PLR | 200 | Low | 124 | 75 | 49 | |||||
| Mitsunaga et al., 2016 [41] | Japan | PC | III-IV | CTx | NLR | 5 | High | 195 | ND | ND |
| Montes et al., 2017 [11] | Spain | PC | III-IV | CTx | NLR | 2.455 | Low | 39 | ND | ND |
| Piciucchi et al., 2017 [12] | Italy | PDAC | ND | Mixed | NLR | 5 | High | 206 | 60 | 144 |
| Shirai et al., 2015 [15] | Japan | PDAC | ND | ND | NLR | 5 | High | 131 | 15 | 116 |
| PLR | 150 | Low | 131 | 73 | 58 | |||||
| Sierzega et al., 2017 [13] | Poland | PDAC | I-III | Mixed | NLR | 5 | High | 442 | 119 | 323 |
| Sugiura et al., 2013 [34] | Japan | PDAC | III-IV | Mixed | NLR | 4 | Low | 83 | 36 | 47 |
| Sugiura et al., 2017 [9] | Japan | PDAC | III-IV | CTx | NLR | 4 | Low | 129 | 62 | 67 |
| Szkandera et al., 2014 [33] | Austria | PDAC | I-IV | Mixed | NLR | 3.25 | Low | 474 | 247 | 227 |
| Tao et al., 2016 [16] | China | PDAC | I-IV | Mixed | NLR | 2.5 | Low | 154 | 84 | 70 |
| PLR | 150 | Low | 154 | 81 | 73 | |||||
| Takakura et al., 2016 [26] | Japan | PC | I-III | Mixed | NLR | 4.3 | High | 28 | ND | ND |
| Teo et al., 2013 [40] | Ireland | PDAC | III-IV | CTx | NLR | 3 | Low | 85 | 58 | 27 |
| Tsujita et al., 2017 [8] | Japan | PC | II-IV | Mixed | NLR | 3 | Low | 86 | ND | ND |
| Vivaldi et al., 2016 [18] | Italy | PC | III-IV | CTx | NLR | 4 | Low | 119 | 21 | 98 |
| Wang et al., 2012 [35] | China | PDAC | I-IV | Mixed | NLR | 5 | High | 177 | 32 | 145 |
| Wu et al., 2016 [17] | China | PDAC | III-IV | Mixed | NLR | 5 | High | 233 | 57 | 176 |
| Xu et al., 2017 [38] | China | PDAC | I-IV | Mixed | NLR | 3.8 | Low | 265 | 71 | 194 |
| PLR | 182.1 | High | 265 | 87 | 178 | |||||
| Xue et al., 2014 [32] | Japan | PDAC | III-IV | CTx | NLR | 5 | High | 252 | 40 | 212 |
| PLR | 150 | Low | 252 | 148 | 104 | |||||
| Yamada et al., 2016 [20] | Japan | PC | I-IV | Mixed | NLR | 3 | Low | 379 | 130 | 249 |
| PLR | 150 | Low | 379 | 192 | 187 | |||||
| Yu et al., 2017 [39] | China | |||||||||
| Training set | PC | III-IV | CTx | NLR | 3.42 | Low | 139 | 93 | 46 | |
| Validation set | PC | III-IV | CTx | NLR | 3.42 | Low | 225 | 131 | 94 | |
Tx: Treatment; PDAC: pancreatic ductal adenocarcinoma; PC: pancreatic cancer; CTx: chemotherapy; RTx: radiation therapy; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; ND: no description. “Mixed” treatment indicates surgery plus chemotherapy/radiotherapy.
3.2. Meta-Analysis
First, the relationships between high NLR and PLR values and PC were investigated. Overall, the rates of high NLR and PLR as determined using the random-effects model were 0.379 (95% CI 0.310–0.454) and 0.490 (95% CI 0.438–0.543), respectively. Additional data, as well as the results of heterogeneity and publication bias analyses, are shown in Table 2. Patients with lower tumor stages (I and II) showed higher NLRs than those with higher tumor stages (III and IV). Additionally, the rate of high NLR was higher in patients who had undergone surgery than in those who received other treatments. There were no differences in the rates of high NLR and PLR between study locations. In comparison between higher and lower criterion subgroups, higher criteria of NLR showed significantly higher rate of high NLR than lower criteria of NLR. However, in PLR, there was no significant difference between higher and lower criteria of PLR.
Table 2.
Estimated rates of high neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios.
| Number of subset | Fixed effect (95% CI) | Heterogeneity test (P value) | Random effect (95% CI) | Egger's test (P value) | |
|---|---|---|---|---|---|
| NLR | |||||
| Overall | 29 | 0.422 (0.409, 0.436) | <0.001 | 0.379 (0.310, 0.454) | 0.086 |
| Location | |||||
| Asia | 22 | 0.426 (0.411, 0.442) | <0.001 | 0.370 (0.285, 0.464) | 0.067 |
| Non-Asia | 7 | 0.413 (0.388, 0.438) | <0.001 | 0.406 (0.295, 0.528) | 0.855 |
| Tumor stage | |||||
| I and II | 1 | 0.656 (0.587, 0.720) | 1.000 | 0.656 (0.587, 0.720) | — |
| III and IV | 14 | 0.406 (0.384, 0.428) | <0.001 | 0.373 (0.273, 0.484) | 0.375 |
| Treatment | |||||
| Surgery | 1 | 0.701 (0.653, 0.745) | 1.000 | 0.701 (0.653, 0.745) | — |
| Chemotherapy | 10 | 0.426 (0.401, 0.451) | <0.001 | 0.403 (0.276, 0.544) | 0.571 |
| Chemo- and radiotherapy | 2 | 0.399 (0.325, 0.478) | <0.001 | 0.258 (0.045, 0.721) | — |
| Mixed | 15 | 0.401 (0.385, 0.418) | <0.001 | 0.380 (0.294, 0.474) | 0.359 |
| NLR criteria | |||||
| High (>4) | 14 | 0.246 (0.230, 0.263) | <0.001 | 0.237 (0.184, 0.299) | 0.650 |
| Low (≤4) | 15 | 0.530 (0.514, 0.547) | <0.001 | 0.533 (0.459, 0.606) | 0.932 |
| PLR | |||||
| Overall | 13 | 0.482 (0.464, 0.500) | <0.001 | 0.490 (0.438, 0.543) | 0.523 |
| Location | |||||
| Asia | 11 | 0.476 (0.457, 0.495) | <0.001 | 0.480 (0.422, 0.539) | 0.751 |
| Non-Asia | 2 | 0.533 (0.480, 0.586) | 0.047 | 0.545 (0.435, 0.651) | — |
| Tumor stage | |||||
| III and IV | 6 | 0.552 (0.522, 0.582) | 0.028 | 0.542 (0.492, 0.592) | 0.209 |
| Treatment | |||||
| Chemotherapy | 3 | 0.569 (0.530, 0.607) | 0.042 | 0.552 (0.478, 0.624) | 0.096 |
| Chemo- and radiotherapy | 2 | 0.555 (0.483, 0.625) | 0.061 | 0.540 (0.400, 0.674) | — |
| Mixed | 7 | 0.444 (0.422, 0.466) | <0.001 | 0.448 (0.387, 0.510) | 0.739 |
| PLR criteria | |||||
| High (>150) | 4 | 0.404 (0.373, 0.435) | <0.001 | 0.435 (0.321, 0.556) | 0.148 |
| Low (≤150) | 9 | 0.518 (0.497, 0.540) | 0.006 | 0.519 (0.482, 0.557) | 0.976 |
NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; CI: confidence interval. “Mixed” treatment indicates surgery plus chemotherapy/radiotherapy.
Next, the correlations between high values of these inflammatory markers and OS rates were investigated. High NLR and PLR values were significantly correlated with poorer OS (HR 1.737, 95% CI 1.502–2.009 and HR 1.143, 95% CI 1.037–1.259, resp.; Table 3). Subgroup analyses based on study location, tumor stage, treatment, and cutoffs were conducted. With respect to NLR, all subgroups except for patients who underwent chemotherapy and/or radiotherapy showed significant correlations between high NLR and a poorer OS; a high NLR had no prognostic role in patients who underwent chemo- or radiotherapy (HR 0.922, 95% CI 0.269–3.162). Patients with high PLR values only showed poorer OS if they were Asian or underwent mixed treatment (i.e., surgery plus chemo/radiotherapy).
Table 3.
Correlation between lymphocyte-associated parameters and survival rate.
| Number of subset | Fixed effect (95% CI) | Heterogeneity test (P value) | Random effect (95% CI) | Egger's test (P value) | |
|---|---|---|---|---|---|
| NLR | |||||
| Overall | 35 | 1.147 (0.116, 1.180) | <0.001 | 1.737 (1.502, 2.009) | <0.001 |
| Location | |||||
| Asia | 26 | 1.129 (1.097, 1.162) | <0.001 | 1.763 (1.470, 2.114) | <0.001 |
| Non-Asia | 9 | 1.544 (1.365, 1.747) | 0.054 | 1.626 (1.360, 1.946) | 0.010 |
| Tumor stage | |||||
| I and II | 1 | 1.859 (1.272, 2.717) | 1.000 | 1.859 (1.272, 2.717) | — |
| III and IV | 17 | 1.733 (1.563, 1.921) | <0.001 | 1.929 (1.509, 2.467) | 0.144 |
| Treatment | |||||
| Surgery | 1 | 1.510 (1.148, 1.986) | 1.000 | 1.510 (1.148, 1.986) | — |
| Chemotherapy | 13 | 1.797 (1.603, 2.015) | <0.001 | 2.043 (1.584, 2636) | 0.061 |
| Chemo- and radiotherapy | 2 | 1.333 (0.917, 1.937) | 0.020 | 0.922 (0.269, 3.162) | — |
| Mixed | 18 | 1.110 (1.078, 1.143) | <0.001 | 1.670 (1.385, 2.013) | <0.001 |
| NLR criteria | |||||
| High (>4) | 18 | 1.954 (1.749, 2.183) | <0.001 | 2.001 (1.602, 2.499) | 0.671 |
| Low (≤4) | 17 | 1.107 (1.075, 1.139) | <0.001 | 1.508 (1.285, 1.770) | <0.001 |
| PLR | |||||
| Overall | 14 | 1.009 (1.007, 1.011) | 0.009 | 1.143 (1.037, 1.259) | 0.008 |
| Location | |||||
| Asia | 11 | 1.009 (1.007, 1.011) | 0.026 | 1.121 (1.010, 1.243) | 0.038 |
| Non-Asia | 3 | 1.217 (1.013, 1.462) | 0.171 | 1.239 (0.968, 1.587) | 0.232 |
| Tumor stage | |||||
| III and IV | 6 | 1.155 (1.002, 1.332) | 0.281 | 1.158 (0.983, 1.364) | 0.828 |
| Treatment | |||||
| Chemotherapy | 3 | 1.053 (0.861, 1.287) | 0.488 | 1.053 (0.861, 1.287) | 0.203 |
| Chemo- and radiotherapy | 2 | 1.282 (0.937, 1.753) | 0.075 | 1.206 (0.674, 2.158) | — |
| Mixed | 8 | 1.009 (1.007, 1.011) | 0.021 | 1.125 (1.009, 1.254) | 0.043 |
| PLR criteria | |||||
| High (>150) | 5 | 1.009 (1.007, 1.011) | 0.001 | 1.219 (0.992, 1.499) | 0.080 |
| Low (≤150) | 9 | 1.105 (1.000, 1.222) | 0.561 | 1.105 (1.000, 1.222) | 0.471 |
NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; CI: confidence interval. “Mixed” treatment indicates surgery plus chemotherapy/radiotherapy.
4. Discussion
In this meta-analysis of 34 studies comprising 7105 patients with PC, we showed that the NLR and PLR constitute novel prognostic markers for predicting the prognosis of patients with PC. To the best of our knowledge, our meta-analysis is the first to investigate this relationship. The results of our meta-analysis demonstrated that high NLR and PLR values were found to be correlated with poor OS in patients with PC.
Although there was no significant difference in PLR among patients in the present series, a high NLR was frequently found in patients with lower tumor stages (I and II) and in those who had undergone surgery. In view of the relatively high likelihood of poor outcomes in patients with PC regardless of stage, patients with lower-stage tumors that are usually limited to the pancreas are most likely to undergo surgical resection [44]. The prognosis is better in patients with lower tumor stage than in those with higher stage [45]. In other types of cancer, a lower tumor stage was associated with lower NLR levels [46, 47]. In our study, the subgroup with low tumor stages (I-II) showed higher rate of high NLR than the subgroup with high tumor stages (III-IV) (0.656 versus 0.373). However, because the subgroup with low tumor stage included only one eligible study, further studies will be needed to obtain the detailed information of PC with low stage. A plausible explanation for our apparently counterintuitive results is that a subset of patients included in the analysis underwent palliative surgery [9, 35–37]. Moreover, the time of obtaining blood samples for measuring neutrophils, lymphocytes, and platelets before treatment might be an important limiting factor. Another possible bias in our data is the presence of biliary sepsis; approximately 56% of PC patients present with obstructive jaundice and are more susceptible to bacterial infection owing to bile duct obstruction [48, 49]. Few studies included in our meta-analysis controlled for biliary infection by excluding patients with this septic condition; therefore, such comorbidities may have influenced our findings [13, 26, 28].
The exact mechanisms between high NLR/PLR values and poor outcomes in patients with PC are unclear. Systemic inflammation plays decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis. Inflammation may enhance tumor initiation through genetic mutations, genomic instability, and epigenetic modifications and can activate tissue repair responses that induce proliferation of premalignant cells and prolong their survival. Inflammation also stimulates angiogenesis, causes immunosuppression, and promotes the formation of tumor-supporting microenvironments that ultimately promote metastasis [50]. The close association between increased systemic inflammatory responses (as assessed by NLR and PLR) and poor prognosis may also be related to cancer cell activation of inflammatory processes. Cancer-related inflammation suppresses antitumor immunity by recruiting regulatory T cells and activating chemokines, resulting in tumor progression. Tumors also secrete vascular endothelial growth factor (VEGF), a vascular permeability factor that induces persistent extravasation of fibrin and fibronectin and continuous generation of the extracellular matrix [51]. Platelets are a critical source of cytokines, especially transforming growth factor-beta as well as VEGF, which can promote cancer progression by enhancing angiogenesis [50–52]. Proinflammatory cytokines such as interleukins 1 and 6 can promote megakaryocyte proliferation; this results in thrombocytosis, which is a negative prognostic marker in several cancers [53–55]. Therefore, inflammatory markers might be an indicator of prognosis. Recently, NLR and PLR have now been investigated as prognostic factors. The measurement of the NLR and PLR is straightforward and convenient and is potentially useful in daily oncologic practice.
Our study found that patients with PC who have high NLR values exhibit poor OS which was consistent with the results in other types of malignancies [46, 47, 56]. Subgroup analysis of NLR stratified by study location, tumor stage, treatment, and threshold criteria also demonstrated that a high NLR had a negative effect on OS except in patients who underwent chemotherapy and/or radiotherapy. The NLR after chemo- and radiotherapy did not correlate with OS. NLR is a relative value that fluctuates depending on neutrophil or lymphocyte changes and may therefore be affected by chemotherapy, radiotherapy, or granulocyte colony-stimulating factor administration [29]. These factors might induce changes in the number of neutrophils or lymphocytes; clinicians should consider these conditions in clinical practice. Several studies demonstrated that postchemotherapy NLR change was an independent prognostic marker [8, 29, 32]. Given potential chemotherapy- or radiotherapy-related toxicities, increased NLR values after treatment may help physicians decide to transfer affected patients to early palliative care, whereas a decrease in the NLR after chemo- or radiotherapy can be considered an early predictor of response to treatment.
High PLR could also predict OS of patients with PC in accordance with other malignancies [46, 47, 56]. However, in subgroup analyses, a high PLR was associated with worse OS only in Asian patients and in those who underwent mixed treatment (surgery plus chemo/radiotherapy). Our meta-analysis demonstrated that NLR is a better predictor of prognosis of patients with PC than PLR, which is also consistent with the results of previous studies [35, 57]. Our analysis may provide important information to support treatment decision-making, including pursuing more aggressive treatments.
There were several limitations in this meta-analysis. First, all of the included studies were retrospective and were thereby more prone to some biases. Second, information about PLR in patients who underwent surgical treatment could not be obtained from the eligible studies. Third, a comparison between pre- and posttreatment inflammatory marker values could not be performed owing to insufficient information.
In conclusion, high NLR and PLR values are useful predictors of worse survival in patients with PC. These parameters can therefore be useful for identifying high-risk patients with PC and for determining individual treatment plans.
Data Availability
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.
Conflicts of Interest
The authors disclosed no financial relationships relevant to this publication.
Authors' Contributions
Dongwook Oh and Jung-Soo Pyo equally contributed to this work.
References
- 1.Ilic M., Ilic I. Epidemiology of pancreatic cancer. World Journal of Gastroenterology. 2016;22(44):9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T., Desseigne F., Ychou M., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.von Hoff D. D., Ervin T., Arena F. P., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New England Journal of Medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2017. CA: a Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Proctor M. J., Morrison D. S., Talwar D., et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. European Journal of Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 6.McMillan D. C. Systemic inflammation, nutritional status and survival in patients with cancer. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 7.Jeong J. H., Kim N. Y., Pyo J. S. Prognostic roles of lymph node micrometastasis in non-small cell lung cancer. Pathology, Research and Practice. 2018;214(2):240–244. doi: 10.1016/j.prp.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Tsujita E., Ikeda Y., Kinjo N., et al. Postoperative neutrophil-to-lymphocyte ratio as a predictor of long-term prognosis after pancreatectomy for pancreatic carcinoma: a retrospective analysis. The American Surgeon. 2017;83(6):610–616. [PubMed] [Google Scholar]
- 9.Sugiura T., Okamura Y., Ito T., et al. Prognostic scoring system for patients who present with a gastric outlet obstruction caused by advanced pancreatic adenocarcinoma. World Journal of Surgery. 2017;41(10):2619–2624. doi: 10.1007/s00268-017-4027-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Yan H., Wang Y., Shi Y., Dai G. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Scientific Reports. 2017;7(1):p. 753. doi: 10.1038/s41598-017-00859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montes A. F., Villarroel P. G., Ayerbes M. V., et al. Prognostic and predictive markers of response to treatment in patients with locally advanced unresectable and metastatic pancreatic adenocarcinoma treated with gemcitabine/nab-paclitaxel: results of a retrospective analysis. Journal of Cancer Research and Therapeutics. 2017;13(2):240–245. doi: 10.4103/0973-1482.181181. [DOI] [PubMed] [Google Scholar]
- 12.Piciucchi M., Stigliano S., Archibugi L., et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. International Journal of Molecular Sciences. 2017;18(4) doi: 10.3390/ijms18040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sierzega M., Lenart M., Rutkowska M., et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Annals of Surgical Oncology. 2017;24(3):808–815. doi: 10.1245/s10434-016-5634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z., Jin K., Guo M., et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Annals of Surgical Oncology. 2017;24(2):561–568. doi: 10.1245/s10434-016-5579-3. [DOI] [PubMed] [Google Scholar]
- 15.Shirai Y., Shiba H., Sakamoto T., et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158(2):360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Tao L., Zhang L., Peng Y., et al. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma: a new hypothesis and a new predictor for distant metastasis. Medicine. 2016;95(39):p. e4932. doi: 10.1097/MD.0000000000004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu M., Guo J., Guo L., Zuo Q. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biology. 2016;37(9):12525–12533. doi: 10.1007/s13277-016-5122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivaldi C., Caparello C., Musettini G., et al. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: patients’ outcome and analysis of prognostic factors. International Journal of Cancer. 2016;139(4):938–945. doi: 10.1002/ijc.30125. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. M., Lee H. S., Hyun J. J., et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World Journal of Gastrointestinal Oncology. 2016;8(7):555–562. doi: 10.4251/wjgo.v8.i7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada S., Fujii T., Yabusaki N., et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer: Glasgow prognostic score is the most reliable parameter. Medicine. 2016;95(18):p. e3582. doi: 10.1097/MD.0000000000003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asaoka T., Miyamoto A., Maeda S., et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16(3):434–440. doi: 10.1016/j.pan.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Asari S., Matsumoto I., Toyama H., et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surgery Today. 2016;46(5):583–592. doi: 10.1007/s00595-015-1206-3. [DOI] [PubMed] [Google Scholar]
- 23.Kou T., Kanai M., Yamamoto M., et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. International Journal of Clinical Oncology. 2016;21(1):118–125. doi: 10.1007/s10147-015-0864-x. [DOI] [PubMed] [Google Scholar]
- 24.Alagappan M., Pollom E. L., von Eyben R., et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. American Journal of Clinical Oncology. 2018;41(3):242–247. doi: 10.1097/COC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 25.Cheng H., Luo G., Lu Y., et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16(6):1080–1084. doi: 10.1016/j.pan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Takakura K., Ito Z., Suka M., et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil–lymphocyte ratio and immunohistochemical analyses of the tumour site. Scandinavian Journal of Gastroenterology. 2016;51(5):610–617. doi: 10.3109/00365521.2015.1121515. [DOI] [PubMed] [Google Scholar]
- 27.Kishi T., Nakamura A., Itasaka S., et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2015;15(6):694–700. doi: 10.1016/j.pan.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Ben Q., An W., Wang L., Wang W., Yu L., Yuan Y. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas. 2015;44(3):1–477. doi: 10.1097/MPA.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 29.Luo G., Guo M., Liu Z., et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Annals of Surgical Oncology. 2015;22(2):670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 30.Inoue D., Ozaka M., Matsuyama M., et al. Prognostic value of neutrophil–lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Japanese Journal of Clinical Oncology. 2014;45(1):61–66. doi: 10.1093/jjco/hyu159. [DOI] [PubMed] [Google Scholar]
- 31.Martin H. L., Ohara K., Kiberu A., Van Hagen T., Davidson A., Khattak M. A. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Internal Medicine Journal. 2014;44(7):676–682. doi: 10.1111/imj.12453. [DOI] [PubMed] [Google Scholar]
- 32.Xue P., Kanai M., Mori Y., et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Medicine. 2014;3(2):406–415. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szkandera J., Stotz M., Absenger G., et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. British Journal of Cancer. 2014;110(1):183–188. doi: 10.1038/bjc.2013.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiura T., Uesaka K., Kanemoto H., Mizuno T., Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Annals of Surgical Oncology. 2013;20(13):4330–4337. doi: 10.1245/s10434-013-3227-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang D. S., Luo H. Y., Qiu M. Z., et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Medical Oncology. 2012;29(5):3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 36.An X., Ding P. R., Li Y. H., et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15(6):516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 37.Chawla A., Huang T. L., Ibrahim A. M., Hardacre J. M., Siegel C., Ammori J. B. Pretherapy neutrophil to lymphocyte ratio and platelet to lymphocyte ratio do not predict survival in resectable pancreatic cancer. HPB. 2018;20(5):398–404. doi: 10.1016/j.hpb.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Shi K. Q., Chen B. C., Huang Z. P., Lu F. Y., Zhou M. T. A nomogram based on preoperative inflammatory markers predicting the overall survival of pancreatic ductal adenocarcinoma. Journal of Gastroenterology and Hepatology. 2017;32(7):1394–1402. doi: 10.1111/jgh.13676. [DOI] [PubMed] [Google Scholar]
- 39.Yu S. L., Xu L. T., Qi Q., et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Scientific Reports. 2017;7, article 45194 doi: 10.1038/srep45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teo M., Sharial M. S. N. M., McDonnell F., Conlon K. C., Ridgway P. F., McDermott R. S. Prognostic role of neutrophil-to-lymphocyte ratio in advanced pancreatic ductal adenocarcinoma: impact of baseline fluctuation and changes during chemotherapy. Tumori Journal. 2013;99(4):516–522. doi: 10.1177/030089161309900413. [DOI] [PubMed] [Google Scholar]
- 41.Mitsunaga S., Ikeda M., Shimizu S., et al. C-reactive protein level is an indicator of the aggressiveness of advanced pancreatic cancer. Pancreas. 2016;45(1):110–116. doi: 10.1097/MPA.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 42.Parmar M. K. B., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Peto R. Experimental survival curves for interval-censored data. Applied Statistics. 1973;22(1):p. 86. doi: 10.2307/2346307. [DOI] [Google Scholar]
- 44.Khorana A. A., Mangu P. B., Berlin J., et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2016;34(21):2541–2556. doi: 10.1200/JCO.2016.67.5553. [DOI] [PubMed] [Google Scholar]
- 45.Kamarajah S. K., Burns W. R., Frankel T. L., Cho C. S., Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a Surveillance, Epidemiology and End Results (SEER) analysis. Annals of Surgical Oncology. 2017;24(7):2023–2030. doi: 10.1245/s10434-017-5810-x. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Liang D., Xu X., et al. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncology Letters. 2017;14(6):6449–6456. doi: 10.3892/ol.2017.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yodying H., Matsuda A., Miyashita M., et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Annals of Surgical Oncology. 2016;23(2):646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 48.Sung J. Y., Costerton J. W., Shaffer E. A. Defense system in the biliary tract against bacterial infection. Digestive Diseases and Sciences. 1992;37(5):689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]
- 49.Porta M., Fabregat X., Malats N., et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clinical and Translational Oncology. 2005;7(5):189–197. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 50.Grivennikov S. I., Greten F. R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 52.Bambace N. M., Holmes C. E. The platelet contribution to cancer progression. Journal of Thrombosis and Haemostasis. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 53.Alexandrakis M. G., Passam F. H., Moschandrea I. A., et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. American Journal of Clinical Oncology. 2003;26(2):135–140. doi: 10.1097/00000421-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda M., Furukawa H., Imamura H., et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Annals of Surgical Oncology. 2002;9(3):287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 55.Voutsadakis I. A. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World Journal of Gastrointestinal Oncology. 2014;6(2):34–40. doi: 10.4251/wjgo.v6.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Z., Zhao X., Lu J., Xue J., Liu P., Mao H. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Archives of Gynecology and Obstetrics. 2018;297(4):849–857. doi: 10.1007/s00404-018-4678-8. [DOI] [PubMed] [Google Scholar]
- 57.Gao Y., Wang W. J., Zhi Q., et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8(51):88835–88844. doi: 10.18632/oncotarget.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.


